Top Essay Writers
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
Simply fill out the order form with your paper’s instructions in a few easy steps. This quick process ensures you’ll be matched with an expert writer who
Can meet your papers' specific grading rubric needs. Find the best write my essay assistance for your assignments- Affordable, plagiarism-free, and on time!
Posted: July 22nd, 2024
Literature review
What is the correlation between the pH and absorption of chemicals with varing concentration?
We get a lot of “Can you do MLA or APA?”—and yes, we can! Our writers ace every style—APA, MLA, Turabian, you name it. Tell us your preference, and we’ll format it flawlessly.
The rationale for chosing this topic is that it can be applied to everyday life, ie. Household items and food and the pH of them. For example, the ‘pH of tothpaste is approximately 9 and the pH of lemon juice is approximately 2.0’3. (ThoughtCo, 2018). The dangers of these household items can be quite severe used in large volumes, however when used in small volumes items such as lemon juice are not very harmful.
Acids
Acids are aqueous or gaseous substances that ‘Dissociate’ separate into simpler substances/atoms or molecules. An example of this is hydrogen chloride, which is a gas that has a ‘Covalent Structure’, this gas dissolves in water and forms hydrogen+ and chlorine– ions. The equation for this would be:
Totally! They’re a legit resource for sample papers to guide your work. Use them to learn structure, boost skills, and ace your grades—ethical and within the rules.
‘HCl(g) + H2O —- > H+(aq) + Cl–(aq)’
Hydrogen chloride gas + Water —- > Hydrogen+ ions + Chlorine– ions.
Starts at $10/page for undergrad, up to $21 for pro-level. Deadlines (3 hours to 14 days) and add-ons like VIP support adjust the cost. Discounts kick in at $500+—save more with big orders!
Hydrgoen was discovered to be an unstable element when it is alone and can simply be defined as a proton. In electrolysis reactions hydrogen ions combine with ‘Polar water molecules’ which in turn will then form stable ions called ‘oxonium ions’. An equation to show this is:
‘H+(aq) + H2O(l) —- > H3O+(aq)
Figure 1
Hydrogen + Water —- > Hydronium (RSC.org, 2018)
100%! We encrypt everything—your details stay secret. Papers are custom, original, and yours alone, so no one will ever know you used us.
Figure 1 shows a diagram which highlights the pH scale and includes written examples of each pH.
(store,2018)
In addition, when hydrogen chloride as an aqueous solution is further dissolved in a volume of water, scientists have speculated that there is a chemical reaction taking place. The equation for the process is:
HCl(aq) + H2O(l) —- > H3O+(aq) + Cl–(aq)
Nope—all human, all the time. Our writers are pros with real degrees, crafting unique papers with expertise AI can’t replicate, checked for originality.
Hydrogen chloride + Water —- > Hydronium + Chlorine (RSC.org, 2018)
The definition of an acid is: ‘A compound containing hydrogen that dissociates in water to form hydrogen ions’ (Annets et al., 2017).
Other common acids also react the same way, they dissociate and separate into simpler substances/ atoms or molcules, the amount they dissociate can vary from greater to lesser, but in water they all form oxonium ions. Therefore, this information explains why acids only portray their acidic properties when they have been diluted into a volume of water. (RSC.org, 2018).
Acids are substances that: form hydrogen gas when reacted with metals, neturalise bases and produce salt and water, and they release carbon dioxide if they are reacted with ‘Carbonates’. (RSC.org, 2018).
Our writers are degree-holding pros who tackle any topic with skill. We ensure quality with top tools and offer revisions—perfect papers, even under pressure.
‘Acids provide H+ ions in a solution’. Acids can therefore be defined or described as ‘proton donors’. From this the assumption can be made that alkalis are ‘proton acceptors’ as they are chemical opposites to acids. (RSC.org, 2018).
Acid dissociation constant
The rate at which an acid dissociates varies with different acids. For example this could mean that more of the acidic molecules produce H+ ions and fhs ‘equilibrium position is further to the right’. An equation to show this for a general acid with the formula HA:
By following ‘Le Chatelier’s principle’, by adding more water to the acid , the ionisation of the acid will increase.
Experts with degrees—many rocking Master’s or higher—who’ve crushed our rigorous tests in their fields and academic writing. They’re student-savvy pros, ready to nail your essay with precision, blending teamwork with you to match your vision perfectly. Whether it’s a tricky topic or a tight deadline, they’ve got the skills to make it shine.
Therefore the ‘acid dissociation constant’ can be shown as this:
However, the concentration of the water being used is unlikely to vary very much as water is a neutral liquid, often around pH 7. Therefore, by taking this into consideration, the water constant is ‘incorperated’ into Kc, which can be shown in this equation:
Guaranteed—100%! We write every piece from scratch—no AI, no copying—just fresh, well-researched work with proper citations, crafted by real experts. You can grab a plagiarism report to see it’s 95%+ original, giving you total peace of mind it’s one-of-a-kind and ready to impress.
The values of
In this equation K can be defined as the ‘acid dissociation consatnt’
The ‘greater the degree of ionisation’ that is found, the stronger the acid will be. Therefore the value of K2 will increase.
Yep—APA, Chicago, Harvard, MLA, Turabian, you name it! Our writers customize every detail to fit your assignment’s needs, ensuring it meets academic standards down to the last footnote or bibliography entry. They’re pros at making your paper look sharp and compliant, no matter the style guide.
When acids have the same concentration, the ‘greater the degree of ionisation’ of the acid being used, the more ions that are present in that acid. Therefore this will mean that the conductivity of the acid is higher. (RSC.org, 2018)
Some examples of acid dissociated constants are:
The reason that the value of Ka for trichloroethanoic acid is 10,000 times bigger than the value of Ka for ethanoic acid is because chlorine is an ‘electronegative atom’, this means that electrons are attracted to the chlorine nucleus. This weakens the O-H bond as the hydrogen ions are much easier to form. The more hydrogen ions found in a solution, the stronger the acidity of the solution.
For sure—you’re not locked in! Chat with your writer anytime through our handy system to update instructions, tweak the focus, or toss in new specifics, and they’ll adjust on the fly, even if they’re mid-draft. It’s all about keeping your paper exactly how you want it, hassle-free.
Some examples of strong acids are sulfuric acid and nitric acids, these are common inorganic acids and they have high values of Ka as they acids are ‘fully ionised in a dilute solution’. (RSC.org, 2018)
pKa = -log Ka (RSC.org, 2018) OR pKa = -log Ka (Chemguide.co.uk, 2018)
The smaller the calculated or written value of pKa , the sronger the acidity of a solution.(RSC.org, 2018)
Ka has the units of: (mol dm-3) whereas, pKa does not have any units. (Chemguide.co.uk, 2018)
It’s a breeze—submit your order online with a few clicks, then track progress with drafts as your writer brings it to life. Once it’s ready, download it from your account, review it, and release payment only when you’re totally satisfied—easy, affordable help whenever you need it. Plus, you can reach out to support 24/7 if you’ve got questions along the way!
pKa is often used as it is easier to identify trends and patterns than using the Ka. (Chemguide.co.uk, 2018)
Examples of strong and weak acids:
Hydrochloric acid is a strong acid as in a dilute solution it is completely dissociated. An equation for this is:
HCl(aq) —- > H+(aq) + Cl–(aq)
Need it fast? We can whip up a top-quality paper in 24 hours—fully researched and polished, no corners cut. Just pick your deadline when you order, and we’ll hustle to make it happen, even for those nail-biting, last-minute turnarounds you didn’t see coming.
Hydrochloric acid —- > Hydrogen + chlorine
However, in comparison both phenol and ethanoic acid can be described as weak acids as they never ‘completely ionised’ and therefore the dilution of the solution does not matter. Equations for both of these weak acids:
Phenol: C6H5H(aq) —- > C6H5O–(aq) + H+(aq)
Ethanoic acid: CH3COOH(aq) —- >CH3COO–(aq) + H+(aq)
There are many weak acids which are produced and occur naturally and therefore their origin is organic, such as:
Absolutely—bring it on! Our writers, many with advanced degrees like Master’s or PhDs, thrive on challenges and dive deep into any subject, from obscure history to cutting-edge science. They’ll craft a standout paper with thorough research and clear writing, tailored to wow your professor.
Strong acids are acids which are fully dissociated into simpler ions in a dilute solution.
Concentrated acids contain ‘serveral moles of substance’ for each dm3 of solution. For example, ‘ordinary lab’ hydrochloric acid has ‘approximately 10 mol dm-3 of HCl(aq). Another example is sulfuric acid which has approximately 18 mol dm-3 of aqueous H2SO4. (RSC.org, 2018)
Alkalis
Alkalis have a pH of 7-14, the higher the pH the stronger the alkalinity is of the solution. Weak alkalis, also referred to as soluble bases, such as ammonia have a pH of 10-11. However, strong alkalis, which are also referred to as soluble bases, such as sodium hydroxide have a pH of 13- 14. These pH levels show as blue/purple colours in solutions when using universal indicator. (Docbrown.info.,2018)
Alkalis form hydroxide ions (OH–(aq)) in water. Such as:
We follow your rubric to a T—structure, evidence, tone. Editors refine it, ensuring it’s polished and ready to impress your prof.
‘An alkali is a base which is soluble in water’. (Docbrown.info.,2018)
Ionic theory of acids and alkalis
Liquid water consists of ‘covalent H2O moleucles’, it also contains small quantities of H+ and OH– ions from the ‘self-ionistion of water’. However, these ions are of equal concentration therefore, this makes the water neutral.
Acidic solutions contain more H+ ions than OH– ions. Alkalis are the opposite to acids, therefore alkali solutions contain more OH– than H+ ions. (Docbrown.info.,2018)
Send us your draft and goals—our editors enhance clarity, fix errors, and keep your style. You’ll get a pro-level paper fast.
When acids and alkalis react the molecular formula equation is often for neutralisation:
Acid+ Alkali —- > Salt + Water Examples:
Hydrochloric acid + Sodium hydroxide —- > sodium chloride + water
However, the ionic equation for neutralisation reacts is:
Hydrogen ion + Hydroxide ion —- > water Which can be shown as:
Yep! We’ll suggest ideas tailored to your field—engaging and manageable. Pick one, and we’ll build it into a killer paper.
H+(aq) + OH–(aq) —- > H2O(l)
This is because all when acids and water are reacted together it produces hydrogen ions. And when alkalis (soluble bases) and water are reacted together they produce hydroxide ions. Therefore, the remaining ions i.e. Na+(aq) and Cl–(aq) become salt crystals i.e. NaCl(s) when the salt ions evapourate from the water. (Docbrown.info.,2018)
‘Bases are substances which react and therefore neutralise acids to produce salts and water.’
Some bases are not soluble in water, examples of this include:
After a neutralisation reaction, the ‘salt solutions consist of a combination of both positive and negative ions’. (Docbrown.info.,2018)
Brownsted-Lowry Theory
In this theory a ‘Brownstead-Lowry acid’ can be referred to as a ‘proton donor’. And as alkalis are the opposite, a ‘Brownstead-Lowry alkali’ can be referred to as a ‘proton acceptor’.
Yes! Need a quick fix? Our editors can polish your paper in hours—perfect for tight deadlines and top grades.
Water is a neutral oxifde as it has a pH of 7, however it is an ‘amphoteric oxide ’ as it accepts and donates protons.
If water acts like a base, a proton acceptor, ‘ reacted with a stronger acid such as the hydrogen chloride gas’, the equation for this is:
HCl(g) + H2O(l) —- > H3O+(aq) + Cl-(aq)
Hydrogen chloride gas + water —- > hydronium + chlorine
If water acts as an acid, a proton donator, reacted with a ‘weal but stronger base such as the alkaline gas ammonia’, the equation for this is:
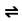 NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) (Docbrown.info.,2018)
NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) (Docbrown.info.,2018)
Sure! We’ll sketch an outline for your approval first, ensuring the paper’s direction is spot-on before we write.
Indicators
‘Indicators are coloured dyestuffs’
There are many types of indicator such as: universal indicator, bromothylmol blue, methyl orange and phenolphthalein. (RSC.org, 2018)
In acids each indicator reacts differently:
In alaklis each indicator reacts differently once again, for example:
Universal indicator is also referred to as a ‘rainbow indicator’ or ‘mixed indicator’ as it as a combination of the single chemical indicators. Universal indicator is often used to find the pH of chemicals and a colour sheet or chart is used to match the colours. Some examples of different substances and the colour they are when universal indicator is added are: (RSC.org, 2018)
A comparison table to show the different indicators and the colour they change a chemical to, dependant on its pH.
(RSC.org, 2018)
| Bromothymol Blue | Methyl orange | Phenolphthalein | Universal indicator/ Mixed indicator | |
| Hydrochloric acid- HCL | Yellow | Red | Colourless | Red/Orange |
| Boric acid- |
You Want The Best Grades and That’s What We Deliver
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
We offer the lowest possible pricing for each research paper while still providing the best writers;no compromise on quality. Our costs are fair and reasonable to college students compared to other custom writing services.
You’ll never get a paper from us with plagiarism or that robotic AI feel. We carefully research, write, cite and check every final draft before sending it your way.