Top Essay Writers
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
Simply fill out the order form with your paper’s instructions in a few easy steps. This quick process ensures you’ll be matched with an expert writer who
Can meet your papers' specific grading rubric needs. Find the best write my essay assistance for your assignments- Affordable, plagiarism-free, and on time!
Posted: September 16th, 2024
Introduction
Monoclonal antibodies, in biomedical research, are used as reagents in diagnosis and treatment of diseases like cancer and infections [1]. It has been almost century their introduction, mAbs are still produced from splenocytes fused to myeloma cells [2]. The antibodies are produced by obtaining cell lines from animals immunized with substance to be studied. To produce the cell lines, B cells obtained from immunized mice are fused with myeloma (immortalized) cells [1][3]. For production of desired monoclonal antibodies, the cells should grow in one of the two ways: injecting the peritoneal cavity of mouse (known as in vivo method or mice ascites method) or by in vitro method (Tissue culture method). Further mouse ascites fluid or supernatant of tissue culture is processed and monoclonal antibody of desired concentration and purity is obtained (figure1) [1]. Mice ascites method is preferred as it is familiar, properly understood and extensively used in laboratories in comparison to tissue culture method which is time consuming, expensive and laborious and ails to give required amount of antibodies[1][3]. Presently, twenty two monoclonal antibodies for transplantation, oncology, infectious, cardiovascular and chronic inflammatory disease have been approved by FDA [3]. Strict guidelines has been setup by IACUC for use of animal for mAb production which includes (i) use of animal is scientifically justified (ii) methods to be used which gives minimum pain to the animal[1].
We hear “Can you write in APA or MLA?” all the time—and the answer’s a big yes, plus way more! Our writers are wizards with every style—APA, MLA, Harvard, Chicago, Turabian, you name it—delivering flawless formatting tailored to your assignment. Whether it’s a tricky in-text citation or a perfectly styled reference list, they’ve got the skills to make your paper academically spot-on.
Monoclonal antibody production (Past to Present)
Mouse mAbs
This technology was introduced in 1975, which works on generation of mouse hybridomas by fusion of B cells, obtained from immunized mice, and myeloma cells. But mAbs produced by this method have many limitations and is not preferred due to high immunogenicity in humans and due to production of human anti-mouse antibody which leads to their rapid clearance from patient’s body [3].
Chimeric mAbs
Yes, completely! They’re a valid tool for getting sample papers to boost your own writing skills, and there’s nothing shady about that. Use them right—like a study guide or a model to learn from—and they’re a smart, ethical way to level up your grades without breaking any rules.
These are produced by gene manipulation method in which constant regions of mouse Abs are replaced by human Abs. Like mouse mAbs, chimeric mAbs also leads to formation of human anti-mouse antibodies and leads to various immunogenicity in patients thus to make it potent in therapeutics further better understanding is required in their structure and function [3].
Humanized mAbs
In this method, complementary determining regions (CDRs) are transferred to human IgG from mouse mAb. There is only 5-10% non-human content in humanized mAbs in comparison to 30% in chimeric mAbs [3].
Prices start at $10 per page for undergrad work and go up to $21 for advanced levels, depending on urgency and any extras you toss in. Deadlines range from a lightning-fast 3 hours to a chill 14 days—plenty of wiggle room there! Plus, if you’re ordering big, you’ll snag 5-10% off, making it easier on your wallet while still getting top-notch quality.
Generation of mAbs
 Immunization of mice
Immunization of mice
 Screening of sera
Screening of sera
Spleen cell isolation Myeloma cells prep.
Nope—your secret’s locked down tight. We encrypt all your data with top-tier security, and every paper’s crafted fresh just for you, run through originality checks to prove it’s one-of-a-kind. No one—professors, classmates, or anyone—will ever know you teamed up with us, guaranteed.
 Cell fusion
Cell fusion
(Tissue culture)
Not even a little—our writers are real-deal experts with degrees, crafting every paper by hand with care and know-how. No AI shortcuts here; it’s all human skill, backed by thorough research and double-checked for uniqueness. You’re getting authentic work that stands out for all the right reasons.
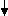 Hybridoma screening
Hybridoma screening
 Selecting cultures for cloning
Selecting cultures for cloning
 Mouse Feeder cells
Mouse Feeder cells
 Cloning (limiting dilution)
Cloning (limiting dilution)
Our writers are Ph.D.-level pros who live for nailing the details—think deep research and razor-sharp arguments. We pair that with top plagiarism tools, free revisions to tweak anything you need, and fast turnarounds that don’t skimp on quality. Your research paper won’t just shine—it’ll set the bar.
 Clone isolation and expansion
Clone isolation and expansion
 Cell freezing and recovery
Cell freezing and recovery
 Supernatant production (from tissue culture media)
Supernatant production (from tissue culture media)
mAbs purification and testing
You’re in good hands with degree-holding pros—many rocking Master’s or higher—who’ve crushed our tough vetting tests in writing and their fields. They’re your partners in this, hitting tight deadlines and academic standards with ease, all while tailoring every essay to your exact needs. No matter the topic, they’ve got the chops to make it stellar.
Figure1. Flowchart showing steps for production of monoclonal antibodies by tissue culture method [1].
Monoclonal Antibody Production Against various Diseases
In this method, S100A4 was used for immunizing female Balb/cAnNHscl mice and mAbs were obtained from fused myeloma and spleen cells using PEG-1500. Hybridomas were selected on HAT medium and further screened for it reaction with S100A4 by ELISA. Clones were selected which were corresponding to 5C3 mAb. Cell culture was scaled up in humid conditions (air 94% and 6% CO2) at 37°C temperature [4]. Supernatant (serum free) from hybidomas was obtained and purified on column containing protein A with the help of AKTA purifier FPLC system and elutions containing 5C3 mAbs were concentrated and filtered in PBS centrifuge Amicon Ultra-15 which has low binding Ultracel membrane and then quantifying mAbs at 280nm [5][6].
Oxytetracycline is used as medication feed in aquaculture [7], its overuse can lead to its accumulation in aquaculture food and its consumption then leads to serious health problems in sea food lovers. To prevent consumers from its harmful effects mAb 2-4F, highly sensitive and specific, were produced for detection of OTC in aquaculture food animals by ELISA. Hybridomas were obtained by standard protocol, by immunizing the female BABL/c mice with OTC-BSA, hybridomas were cultured and supernatants from culture were screened for antibodies using iELISA and antibodies were cloned by limiting dilution method to obtain monoclones then in serum free media these moloclones were cultured in 500 ml spinner flask [6][7]. Further mAbs were purified from this culture using protein G by affinity chromatography. The elute fractions were collected and its protein concentration was determined at 280nm spectometrically and mAb was filtered using cellulose acetate membrane (0.2 µm) and kept at -20°C until used [8].
100%—we promise! Every paper’s written fresh from scratch—no AI, no copying—just solid research and proper citations from our expert writers. You can even request a plagiarism report to see it’s 95%+ unique, giving you total confidence it’s submission-ready and one-of-a-kind.
Interleukin-21 is a type I cytokine with four helical bundles that exerts effect on hematopoietic cells like NK cells, T and B lymphocytes. CD4+ T and NK T cells produce interleukin-2 cytokine, over expression of IL-2 lead to variety of autoimmune disorders. Genetically modified Kirin-Medarex mice were immunized with rhIL-21, immunogens were emulsified with P-adjuvant and CpG and recombinant mouse GM-CSF. Hybridomas obtained were cultured in IMDM containing 1x GlutMax, 1x Penicillin, 10% fetal clone serum and 10% Hybridoma Cloning Factor. Hybridomas were selected with IMDM in conjugation with HAT medium and cloning was carried out with 1x HT and distributed in 96 well Elisa plate and wells were examined microscopically for monoclonality and screened with phosphorylated-STAT3. Wells with positive results were distributed in 24 well cultures to obtained density 6 105 cells/ml and then supernatant was collected and cells cryopreserved. Further media with human IgG was obtained and filtered through 0.2µm membrane and from this filtered media antibody protein was purified by combing Protein G Sepharose Affinity Chromatography Size Exclusion Chromatography and absorbance was taken at 280nm and further its quality was accessed by size exclusion HPLC [9].
105 cells/ml and then supernatant was collected and cells cryopreserved. Further media with human IgG was obtained and filtered through 0.2µm membrane and from this filtered media antibody protein was purified by combing Protein G Sepharose Affinity Chromatography Size Exclusion Chromatography and absorbance was taken at 280nm and further its quality was accessed by size exclusion HPLC [9].
The α-galactosylceramide also known as KRN7000 is best studied ligand that binds to protein CD1d. KRN7000:mCD1d complex is easily recognized by iNKT cells and leads to number of proinflammatory and immunoregulatory functions. To understand the mechanism of antigen presentation to CD1d by iNKT cell three monoclonal antibodies L317, L3363, and L386 were produced. Primary immunogen was prepared with protein obtained from strain H37Ra of Mycobacterium tuberculosis (PPD) and it was conjugated with the complex KRN7000:CD1d. The complex KRN7000:mCD1d:PPD was studied by SDS-PAGE. Mice were first vaccinated with Mycobacterium bovis (BCG) then after 23 days mice were immunized with 5µg KRN7000:CD1d:PPD complex in 1:1 PBS and Imject alum. At day 61 booster dose was given to mice, of the complex, with 7 106 cells.
106 cells.
Mice were then sacrificed and spleens dispersed PBS, cells were obtained and further washed with PBS and erythrocytes were lysed and cells were suspended in FBS/HEPES free DMEM [10][11]. The preparation was then mixed with myeloma cells and centrifuged and tubes with pellet were placed in water bath set at 40°C and into this heated PEG was added followed by FBS/HEPES- free DMEM and then cells again centrifuged and re-suspended in DMEM. Hybridomas along with MRC-5 fibroblast feeder blast cells were plated in 96 well tissue culture plates. Supernatant from culture was screened and cloning of hybridomas carried out by limit dilution. Then 108 cells were inoculated in 2 liters roller bottles containing 500ml medium and OptiMAb supplement was added. MAbs were obtained by filtering of supernatant through protein G column chromatography [12].
Shiga Toxin 2 also designated as Stx2 is virulence causes gastrointestinal disease in humans’ world by food poisoning. It subtype Stx2f cannot be easily detected by immunological methods and thus three monoclonal antibodies specific to it were produced. Complete hybridoma media contains Iscove’s modified DMM with NaHCO3 and 1 Glutamax, containing fetal calf serum (heat inactivated) [13]. Female Balb/cJ mice were immunized with His-tagged Stx2f and hybridomas were obtained and screened for antibodies against Stx2f by ELISA and were further transferred to MPCM/HT/cHM media and diluted 500cells/ml and then the cells were grown in cHM media. Media containing antibody (400ml) was filtered through protein G column and elution were obtained in 0.1M glycine giving 5mg of purified antibody Stx2f [14][15].
Glutamax, containing fetal calf serum (heat inactivated) [13]. Female Balb/cJ mice were immunized with His-tagged Stx2f and hybridomas were obtained and screened for antibodies against Stx2f by ELISA and were further transferred to MPCM/HT/cHM media and diluted 500cells/ml and then the cells were grown in cHM media. Media containing antibody (400ml) was filtered through protein G column and elution were obtained in 0.1M glycine giving 5mg of purified antibody Stx2f [14][15].
Yep—APA, Turabian, IEEE, Chicago, MLA, whatever you throw at us! Our writers nail every detail of your chosen style, matching your guidelines down to the last comma and period. It’s all about making sure your paper fits academic expectations perfectly, no sweat.
EB66 cell lines are derived from embryonic stem cells of duck which can be genetically engineered and production of mAbs can be increased above 1g/L when grown in serum free media. EB66 have various other characteristic features like short doubling time, high cell density and unique metabolic profile with low accumulation of ammonium and lactate and low consumption of glutamine [16]. Further, EB66 cell lines used for production of mAbs has reduced fucose content with enhanced ADCC activity. EB66 cell lines produce chimeric IgG1 anti-cancer mAb against antigen anti-X by nucleofection. EB66 clones when grown in Erlenmeyer flask with standard fed batch culture produces 1.28g/L of IgG1 of cell density with 36 millions cells/ml. Further by accumulation of monoclonal antibodies in supernatant culture no degradation was observed in antibody production assessed by HPLC, SDS-PAGE and western blot. When the supernatant was purified with Protein-A HPLC showed 98% mAbs as monomers. Glycosylation profile of monoclonal antibodies was analyzed by MALDI-TOF-MS, enhanced activation of the monoclonal antibodies obtained from EB66 cell lines was analyzed by flow cytometry[16][17].
FDA Approved mAbs in market [18][19]
|
Generic Name Trade name Target |
Infliximab Remicade® TNF
Absolutely—life happens, and we’re flexible! Chat with your writer anytime through our system to update details, tweak the focus, or add new requirements, and they’ll pivot fast to keep your paper on point. It’s all about making sure the final draft is exactly what you need, no stress involved.
Rituximab Rituxan®, MabThera® CD20
Trastazumab Herceptin® HER2
Bevacizumab Avastin® VEGF
Adalimumab Humira® TNF
It’s super easy—order online with a few clicks, then track progress with drafts as your writer works their magic. Once it’s done, download it from your account, give it a once-over, and release payment only when you’re thrilled with the result. It’s fast, affordable, and built with students like you in mind!
Cetuximab Erbitux® EGFR
Ranibizumab Lucentis® VEGF
Palivizumab Synagis® RSV
Tositumomab Bexxar® CD20
We can crank out a killer paper in 24 hours—quality locked in, no shortcuts. Just set your deadline when you order, and our pros will hustle to deliver, even if you’re racing the clock. Perfect for those last-minute crunches without compromising on the good stuff.
Alemtuzumab Campath® CD52
Certolizumab pegol Cimiza® TNF
Gemtuzumab ozogamicin Mylotarg® CD33
Muromonab-CD3 Orthoclone Okt3® CD3
Efalizumab Raptciva® CD11a
For sure! Our writers with advanced degrees dive into any topic—think quantum physics or medieval lit—with deep research and clear, sharp writing. They’ll tailor it to your academic level, ensuring it’s thorough yet easy to follow, no matter how tricky the subject gets.
Abciximab ReoPro® GP IIb/IIIa
Basiliximab Simulect® CD25
Eculizumab Soliris® C5
Natalizumab Tysabri® a-4 integrin
Panitumumab Vectibix® EGFR
We stick to your rubric like glue—nailing the structure, depth, and tone your professor wants—then polish it with edits for that extra shine. Our writers know what profs look for, and we double-check every detail to make sure it’s submission-ready and grade-worthy.
Omalizumab Xolair® IgE
Daclizumab Zenapax® CD25
Ibritumomab tiuxetan Zevalin® CD20
Recent advances in mAbs production
Engineered Monoclonal antibodies
Advancement in mAb engenrreing has lead to transformation in this field which has lead to production of new drugs which as many useful characteristics like decreased immunogenicity, improved specifity along with stability and potency [18]. The replacements of murine as well as chimeric mAbs with full human mAbs are boon of this novel technology for example adalimumab, ranibizumab and cetrolizumab pegol. Adalimumab, the human mAb, is created by using phage display technology and now it is the top selling drug in the market. Cetrolizumab pegol has been engineered to increase its half-life by making changes in its Fab fragments [19]. Ranibizumab which is derived from bevacizumab wet AMD (age-related macular degeneration) and is considered as care indication standard. These new engineered mAbs have potential to compete with the drugs already in market and have bright future ahead [19][20].
Biosimalar Monoclonal antibodies
Biosimilars are the copies of drugs whose patient has expired and now these drugs can be produ-
Yes—we’ve got your back! We’ll brainstorm fresh, workable ideas tailored to your assignment, picking ones that spark interest and fit the scope. You choose the winner, and we’ll turn it into a standout paper that’s all yours.
-ced and manufactured by any company. But due to complex molecule used and then its approval from U.S makes it a complex process therefore most of the biotechnology companies are not in favor of production of biosimilars. Dr. Reddy in India has launched Reditux® which is anti-CD20 monoclonal antibody and it is claimed, as the first biosimilar monoclonal antibody, by the company. In spite of approval of Reditux® in India, it is thought that it would not have sufficient data that can fulfill the set standards of developed countries in terms of strict safety, efficacy and manufacturing standards[18][19][20].
Conclusion
Monoclonal antibodies are expanding rapidly in pharmaceutical industries with already hundreds of candidates are under development and trials. Both cytotoxic and radiology methods are emerging to increase efficacy of the present therapeutic molecules. Moreover, advances have also been made to use mAbs in treatment of bacterial and viral infection. Biosimilars and bio-superiors are the next generation drugs which can be produced as most of the blockbuster monoclonal antibody are at verge to their patent expiry. The future of the monoclonal antibodies in therapeutics is bright and continued discovery, research and development in this field can take it to the heights that have not been achieved before.
Abstract
Monoclonal antibodies today have gained a breakthrough and are used in treatment of numbers of disease. Over 30% of the Engineered Monoclonal antibodies are under clinical trials. Moreover, different methods to generate human monoclonal antibodies are present today like generation of humanized and chimeric antibodies from genetic engineering of mouse antibodies, phage display method and transgenic mice development. Monoclonal antibodies are in great demand today and FDA has approved almost 22 mAbs till date and all these are commercially available in market. Biosilimars are also taking up the pace as most of the blockbuster mAbs are at verge of their patient expiry and Reditux® developed by Dr. Reddy claimed as first biosimilar in India and is half the cost of Rituximab®.
Tags: Best Essay Writing Website for PhD Essays, Cheap Dissertation Writer USA and UK, Custom Essay and Assignment Writing Australia, Doctoral Dissertation Writing Service ChinaYou Want The Best Grades and That’s What We Deliver
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
We offer the lowest possible pricing for each research paper while still providing the best writers;no compromise on quality. Our costs are fair and reasonable to college students compared to other custom writing services.
You’ll never get a paper from us with plagiarism or that robotic AI feel. We carefully research, write, cite and check every final draft before sending it your way.