Top Essay Writers
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
Simply fill out the order form with your paper’s instructions in a few easy steps. This quick process ensures you’ll be matched with an expert writer who
Can meet your papers' specific grading rubric needs. Find the best write my essay assistance for your assignments- Affordable, plagiarism-free, and on time!
Posted: October 19th, 2022
In 1871 Harry Brearley was born and he was in in charge of a research laboratory in Sheffield, England in 1907. Gun barrels at that time had a tendency to rust, therefore it was being investigated at the lab as to what the reason was for this rusting taking place. There were samples with different amount of alloys. One of the sample which had been discarded from a previous test was not rust while all the others were. It was found that this sample of steel had been alloyed with chromium. At that time this alloy was the most rust resistant than any other metal at that time. As After two months passed after this discovery, for the first time stainless steel was made. [1]
We hear “Can you write in APA or MLA?” all the time—and the answer’s a big yes, plus way more! Our writers are wizards with every style—APA, MLA, Harvard, Chicago, Turabian, you name it—delivering flawless formatting tailored to your assignment. Whether it’s a tricky in-text citation or a perfectly styled reference list, they’ve got the skills to make your paper academically spot-on.
Brearley wanted to sell his new invention after it being cast. At that time Sheffield was known as the city of cutlery manufacturers. Steel of the cutlery was plated with silver or nickel to prevent rusting and this material, stainless steel, seemed to be a perfect replacement for it. As this was a new material, the manufacturers would not manufacture it there Brearley had one them make him knives. These products when marketed were not success full. In 1924, W.H.Hatfield patented the 18-8 stainless steel and was going to become one of the most popular and most used steel. [1]
There have been a lot of changes made in the modern world due to stainless steel. It is also being used more than other metals such as aluminium, carbon steel and carbon. The main reasons being that stainless steel has physical and chemical properties which are useful in every aspect of manufacturing. It has a high corrosion resistance and it also has a high heat resistance. It does not rust and can be formed or welded easily. Stainless steel does not react readily with many other substances and is cheaper than other metals of similar properties. After the invention, as years passed, metallurgist have discovered more methods to make this steel and also ways to control its properties. Stainless steel can be fully recycled and it does not degrade when it is reprocessed. As it is resistant to corrosion and has a long service life, it does not require to be replaced or repaired repeatedly. [2]
As years passed by, a lot of different properties of stainless steel were being discovered. The achievements that were made in the past century regarding stainless steel are summarized in the points below.
Different types of steels that have a good resistance to corrosion are known generally as stainless steels. All of the different types of stainless steels have a minimum of 10.5 percent amount of chromium. Chromium is the alloying element that gives the stainless steel its basic corrosion resistance and also the resistance of the stainless steel to oxidation. Other elements like nickel and molybdenum are also added to improve the corrosion resistance of the stainless steel. Stainless steel has been success in its various uses due to one unique advantage. The alloy chromium in the stainless steel has an attraction for the element oxygen and forms a film of chromium oxide on the stainless steel surface at a molecular level. This film that is formed is very thin and it is a passive layer meaning that it does not react to other materials. It also stays on the layer of steel and does not transfer anywhere else. This layer is also self-renewing therefore if it damaged, it will react to form more chromium oxide. This means that if a material that is made of stainless steel is used for a period of time, it might wear out due to regular use but the material will remain stainless.
Yes, completely! They’re a valid tool for getting sample papers to boost your own writing skills, and there’s nothing shady about that. Use them right—like a study guide or a model to learn from—and they’re a smart, ethical way to level up your grades without breaking any rules.
Stainless steel is also the most cost efficient material to use in a lot of cases. Due to its higher cost, the production cost is more and the experience required when processing it is also greater than that of normal steel but it has better life cycle costs which make it a very attractive material. Stainless steel has a longer service life due to which lesser costs of maintenance is achieved for the applications made using it. Stainless steel can also be fully recycled and also have a high value for its scrap when being decommissioned. [3, 4]
As years passed, many grades of stainless steel were discovered. These grades are in the four main categories of stainless steel which are
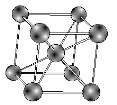 i) Ferritic stainless steels consist of a body centred cubic crystal structure (figure 1) which is similar to that of pure iron at room temperature. The alloying element mainly used is chromium which is between 11 to 17 percent. It also has low carbon which is the reason for its limited strength.
i) Ferritic stainless steels consist of a body centred cubic crystal structure (figure 1) which is similar to that of pure iron at room temperature. The alloying element mainly used is chromium which is between 11 to 17 percent. It also has low carbon which is the reason for its limited strength.
Prices start at $10 per page for undergrad work and go up to $21 for advanced levels, depending on urgency and any extras you toss in. Deadlines range from a lightning-fast 3 hours to a chill 14 days—plenty of wiggle room there! Plus, if you’re ordering big, you’ll snag 5-10% off, making it easier on your wallet while still getting top-notch quality.
When heat treated, ferritic stainless steel can’t be hardened. In comparison with austenite stainless steel, they are cheaper as they do not contain the alloy nickel. They have less toughness which is its ability to deform plastically without it being fractured. It cannot be stretch formed due to low ductility. Compared to austenite stainless steel they have poor weld toughness. This steel is magnetic. [5]
ii) The second grade known is known as the martensitic grade. The structure of this type of 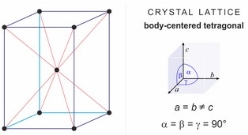 steel consists of body centred tetragonal crystal lattice as shown on figure 2. This type of steel is almost the same as ferritic type of steel regarding the amount of Chromium in it but it has high levels of carbon. They contain at least 12 to 15 percent of chromium. This is the reason that they are harden able and can be tempered similarly to carbon and low alloy steels. It is used when strength is needed and normal corrosion resistance is required. This steel is magnetic. [6, 8]
steel consists of body centred tetragonal crystal lattice as shown on figure 2. This type of steel is almost the same as ferritic type of steel regarding the amount of Chromium in it but it has high levels of carbon. They contain at least 12 to 15 percent of chromium. This is the reason that they are harden able and can be tempered similarly to carbon and low alloy steels. It is used when strength is needed and normal corrosion resistance is required. This steel is magnetic. [6, 8]
Nope—your secret’s locked down tight. We encrypt all your data with top-tier security, and every paper’s crafted fresh just for you, run through originality checks to prove it’s one-of-a-kind. No one—professors, classmates, or anyone—will ever know you teamed up with us, guaranteed.
Precipitation hardened stainless steels are steels which consist of chromium and nickel alloys which provide a good combination of properties martensitic and austenite grade steels. After being heat treated, there strength is increased similarly to the martensitic grade but they also have a corrosion resistance similar to that of austenite stainless steel. [9]
iii) The third grade of stainless steel is known as the austenite grade. This grade of steel is the most commonly used one. When the three elements nickel, manganese and nitrogen are added then this microstructure of austenite steel is derived. This structure gives the steel its ability to be welded and formed. They have a good resistance to corrosion.
These grades of stainless steels have a face centred cubic crystal structure which means that they have one atom at each corner of the cube and one is in the middle of each side. In a steel with 18 percent of chromium alloy at least 8 to 10 percent nickel alloy needs to be added for this grain structure to be formed.
Austenite steels are not magnetic and cannot be heat treated. They can be cold worked which will its hardness, strength and stress resistance. Solution annealing will make the alloy go back to its original condition.
Not even a little—our writers are real-deal experts with degrees, crafting every paper by hand with care and know-how. No AI shortcuts here; it’s all human skill, backed by thorough research and double-checked for uniqueness. You’re getting authentic work that stands out for all the right reasons.
Austenite steels which have a nickel alloy added to them are known as the 300 series. The most common type of steel in this series is 304 stainless steel which contains 8 percent nickel and 18 percent chromium. This is the minimum amount of nickel that can be added to fully convert the steel from ferritic to austenite. Two percent of the alloy molybdenum is added to make the steel more corrosion resistance and the steel is known as 316.
Nitrogen is also used to make austenite steel. The 200 series is the one with low nickel alloy content and high nitrogen content. Only a limited amount of nitrogen can be added because if high amount of it is added then nitrides are formed which makes the alloy weak. However if manganese alloy is added then higher amounts of nitrogen gas can be added and these two elements combined with copper are usually used to replace nickel in the 200 series of stainless steel. [6, 7]
v) The fourth grade of stainless steel is the Duplex type of steel. This grade of stainless steel consist of chromium, nickel, molybdenum, copper and iron. They are known as duplex as they have a 2 phase microstructure which consists of the grains of both ferritic and austenitic type of stainless steel. When this steel is melted, it changes from a liquid to a solid phase of a ferritic structure. As it is cooled to room temperature, half of the ferritic structure gains are transformed to austenitic grains. This type of steel has properties which is a combination of the properties of both the ferritic and austenitic stainless steel. This steel is two times stronger than an austenite or ferritic type of stainless steel. They have better toughness and ductility then the ferritic types of stainless steel but not as well as that of the austenite type of stainless steel. Their resistance to stress corrosion is very good. This is a property taken from the ferritic side of stainless steel. Duplex steels have low amounts of nickel and molybdenum alloys compared to that of austenite stainless steel with the same amount of corrosion resistance. Due to this reason, the cost of duplex steel is low. They are ferromagnetic and are also weld able. There thermal expansion lies between that of the austenite and ferritic type of stainless steel and its other thermal properties are similar to those of plain carbon steels. [10, 11]
Stainless steel has a lot of benefit which can be seen below;
Our writers are Ph.D.-level pros who live for nailing the details—think deep research and razor-sharp arguments. We pair that with top plagiarism tools, free revisions to tweak anything you need, and fast turnarounds that don’t skimp on quality. Your research paper won’t just shine—it’ll set the bar.

 Every day people come into contact with stainless steel either if it is in the form of cutlery or some other material. However in the case of engineering applications, stainless steel is a specialist type of material and is only used when it is vitally required. This is due to the fact that it is a much more difficult material to machine and also has a higher cost in comparison to carbon steels and aluminium. In other types of applications such as in the food, pharmaceutical and chemical industries it is important to use stainless steel because of its resistance to corrosion. As there are a lot of different grades and types of stainless steels available, engineers who do not have essential knowledge about them can easily be confused therefore it is very important that the correct type of stainless steel is used to create the required material so it can also be manufactured at a cost that is reasonable. Stainless steel is different from carbon steels as it as a 10.5 percent of chromium by weight and up to 26 percent can be added to increase the corrosion resistance of the stainless steel in environments that are harsher. The chromium forms an oxide layer by reacting with the air and if it is damaged, the chromium that is exposed forms another layer therefore protecting the metal.
Every day people come into contact with stainless steel either if it is in the form of cutlery or some other material. However in the case of engineering applications, stainless steel is a specialist type of material and is only used when it is vitally required. This is due to the fact that it is a much more difficult material to machine and also has a higher cost in comparison to carbon steels and aluminium. In other types of applications such as in the food, pharmaceutical and chemical industries it is important to use stainless steel because of its resistance to corrosion. As there are a lot of different grades and types of stainless steels available, engineers who do not have essential knowledge about them can easily be confused therefore it is very important that the correct type of stainless steel is used to create the required material so it can also be manufactured at a cost that is reasonable. Stainless steel is different from carbon steels as it as a 10.5 percent of chromium by weight and up to 26 percent can be added to increase the corrosion resistance of the stainless steel in environments that are harsher. The chromium forms an oxide layer by reacting with the air and if it is damaged, the chromium that is exposed forms another layer therefore protecting the metal.
Stainless steel is corrosion resistance and this property gives it the advantage of being readily recyclable. Carbon steels might be very badly corroded and also be contaminated with finishes of paint or other metal plating due to which it cannot be full recycled. Stainless steel is a 100 percent recyclable. In everyday materials made of stainless steel, it is estimated that at least 60 percent of it was recycled stainless steel.
Figure 4 [31] stainless steel parts being manufactured
You’re in good hands with degree-holding pros—many rocking Master’s or higher—who’ve crushed our tough vetting tests in writing and their fields. They’re your partners in this, hitting tight deadlines and academic standards with ease, all while tailoring every essay to your exact needs. No matter the topic, they’ve got the chops to make it stellar.
Stainless steel can be processed into many different types of shapes and sizes to suit the type of applications for it to be used for such has strips, sheets, plates, bars, wires and also tubes as show in Figure 3 and 4. [13]
Architecture and construction
The first time that stainless steel was being used widely in construction was during the art-deco period. The upper portion of the Chrysler building that was the tallest building in 1930 was constructed from stainless steel. Stainless steel is corrosion resistance, flexible and also has high strength due to which it is very commonly used in modern construction. It is used to cover the outer structures of high impact buildings and it also used in the interiors for the handrails, counter tops and also backsplashes.
Stainless steel can be easily welded and it also has a very attractive finish while having low maintenance costs. This is the reason that it is used in modern architecture such as the Eurostar terminal which is in the London’s Waterloo station, the Helix Bridge in Singapore and also the one world trade centre in New York. Green building is the practice in which there is an increase of efficiency through which the buildings and there sites use water, energy and materials. Stainless steel is favoured to use in the building of these structures as it consists of recycled metal. Polishing the stainless steel will also help bring the natural light inside the building which in turn would also reduce the energy consumption. [14]
100%—we promise! Every paper’s written fresh from scratch—no AI, no copying—just solid research and proper citations from our expert writers. You can even request a plagiarism report to see it’s 95%+ unique, giving you total confidence it’s submission-ready and one-of-a-kind.
Automotive and transportation
The first time that stainless steel was used in the automotive industry was in the 1930s by Ford motors to make different concept cars. Presently the use of stainless steel in this industry is growing. Stainless steel is used to make the exhaust systems in the cars, trims and frills and various other parts. The introduction of new reduction standards for the emissions also environment concerns has caused the manufactures to favour stainless steel in the making of structural components too.
Stainless steel is also used in many other types of transportation such as tankers, ship containers and refuse vehicles. It is very good for the transportation of chemicals, liquids and food products as well. Stainless steel has high strength therefore the containers can be made thinner which in turn would save the fuel costs. The corrosion to resistance of the stainless steel would also help reduce the maintenance costs and the cleaning required. [14]
Medical
Yep—APA, Turabian, IEEE, Chicago, MLA, whatever you throw at us! Our writers nail every detail of your chosen style, matching your guidelines down to the last comma and period. It’s all about making sure your paper fits academic expectations perfectly, no sweat.
Stainless steel is also suitable for environments that require hygiene as it does not corrode and as it can be sterilized easily. It is used to make dental and surgical instruments, dishes for kidneys, tables used for operation and also various other types of medical equipment’s like cannulas, steam sterilizers and MRI scanners.
Stainless steel is used in surgical implants and also in different types of replacement joints such as artificial hips. Pins and plates made of stainless steel are used to fix the bones that are broken in their place. [14]
Energy and heavy industries
Different types of industries such as the chemical, gas and oil industries operate in environments that consist of high temperatures and substances that are toxic. Specific grades of stainless steel have been tailored specially for use in these industries which have higher corrosion resistance over a higher range of temperatures. Stainless steel of a high grade is required for the construction of tanks for storage, valves pipes and other components.
Absolutely—life happens, and we’re flexible! Chat with your writer anytime through our system to update details, tweak the focus, or add new requirements, and they’ll pivot fast to keep your paper on point. It’s all about making sure the final draft is exactly what you need, no stress involved.
Stainless steel is all compulsory for oil rigs that are off-shore. Crude oil is very corrosive therefore they have to be constructed from stainless steel which has less weight and is tough. Other technologies that are renewable such as solar, hydro, geothermal and wind power also consist of different parts that are made of stainless steel as it is able to stay as it is in the sea water environments which is highly corrosive. [14]
Food and catering
Stainless steel is also used to manufacture kitchen accessories, cutlery and cookware. The grades which have less ductility are used to make the sharp blades of knives while the grades of stainless steel with higher levels of ductility are used to make materials that have to be moulded into shape such as cookers, sinks, saucepans and grills. Stainless steel is also used to make parts for refrigerators, freezers and dishwashers.
Stainless steel does not have any effect on the food that it comes into contact with therefore it is ideal for food production. The resistance to corrosion of stainless steel is also important as some foods like orange juice can be acidic. Stainless steel can be easily cleaned which prevents germs from staying on the surface of the material. It is also very important in the production of ice cream as it is not effected when strong anti-bacterial cleaning products are used. [14]
It’s super easy—order online with a few clicks, then track progress with drafts as your writer works their magic. Once it’s done, download it from your account, give it a once-over, and release payment only when you’re thrilled with the result. It’s fast, affordable, and built with students like you in mind!
In this era, there are a lot of metals that are being used and of them all, stainless steel plays a very important role. It is also the most common type used for biomedical applications. Care has to be taken when surgical implants are being made as they have to perform the same way they were designed as they are to be implanted inside the human body therefore they will be in contact with parts of the body which could be sensitive to the type of metal and there will be a lot of chances for the immune system to attack the foreign implant. It is very challenging to design and fabricate a medical device meeting all the requirements and with so many applications, medical devices come in all shapes and sizes all depending on the type of job they are being made for. With such an excessive amount of materials, scientists and engineers from different fields are employed to help meet the specific designs that are required. [15]
Stainless steel is the type of steel that is the most commonly used one in the manufacturing of medical devices. Stainless steel 304 from the 300 series of the Austenite grade of stainless steel is used for making medical devices that are used in all types of medical applications. It is the most common type of stainless steel used in the world. Out of all the grades of stainless steel, this is the one that comes in many forms and finishes while having a lot of applications. It is also selected compared to other metals due to its price and therefore is the most logical choice for medical device application. If those medical applications require a stainless steel that is harder or stronger, then the stainless steel can be work hardened through cold working. When they are in there annealed state, they are extremely ductile and can be formed, bent, deep drawn or fabricated with ease. Stainless steel 304 hardens quickly therefore it may require further annealing to improve ductility. [15]
Stainless steel 304 is highly resistant to corrosion and also has a low carbon content which makes it more suitable for medical application than other grades of stainless steel. In hospitals, surgeries and paramedic applications, stainless steel 304 is the best type of material to use as it does not react chemically to the body tissue and it also does not react with the cleaning products that are used to sterilize it. Also not being effected by its hard and continuous use makes it even more suitable. Stainless steel 304 has a low yield strength and also a high elongation potential therefore it ca be formed into complex shapes with being annealed which is heating a metal to a high temperature and then cooling it slowly. [15]
The stainless steel grade 304 has an excellent combination of resistance to corrosion and the ability to be easily formed or shaped. This is the reason due to which these stainless steel alloys are used widely and almost half of the total stainless steel production in the U.S consists of these alloys. The stainless steel grade 304 is available in a range of different types of product forms such as sheets, strips, foils and plates. These stainless steel grade 304 alloys are used to manufacture different types of equipment’s depending on their specific uses. Examples include food, beverage, sanitary, cryogenic, and pressure-containing applications. Previous users using the stainless steel grade 302 have also started to use the stainless steel grade 304 as the argon oxygen decarburization process which provides a way to manufacture stainless steel without losing precious elements has made low level of carbons more easy to attain and also more economical. Sometimes other stainless steel alloys are preferred for example in temper rolled products as the higher level of carbon present allows the requirements of yield strength and tensile strength to be met while having a higher level of ductility. The lower carbon version of stainless steel 304 is used for products that are welded and might not be protected from conditions consisting of intergranular corrosion. The stainless steel grade 304 provides with very useful resistance against corrosion in a wide range of environments which are oxidizing or reducing. These alloys are used a lot in equipment’s which require the handling or processing of food, beverages and dairy products as well. Other type of products which come into contact with water such as heat exchangers, pipes, tanks and other process equipment’s also make use of these alloys. The use of these alloys is also extensive in in architectural and structural applications which are exposed to the atmosphere. [16]
We can crank out a killer paper in 24 hours—quality locked in, no shortcuts. Just set your deadline when you order, and our pros will hustle to deliver, even if you’re racing the clock. Perfect for those last-minute crunches without compromising on the good stuff.
A lot of the applications of the stainless steel grade 304 involve those consisting of household and industrial chemicals. The percentage of the chromium content that these alloys contain provides immunity against environments that are oxidizing such those consisting of dilute nitric acid. When these stainless steel alloys are exposed to temperatures between 800 to 1500 degrees Fahrenheit, precipitation of chromium carbides may occur in the grain boundaries. These grades become sensitized and when they are exposed to aggressive environments, intergranular corrosion occurs. The carbon content that is present in the stainless steel grade 304 may allow it to be sensitized from the high thermal conditions that may arise when the alloy is being welded and from the heat affected zones of the welds. When these conditions are to be experienced, the low carbon version of the same alloy is used which increases the time required for a harmful level of chromium carbides to be precipitated although this does not completely stop the precipitation reaction from occurring in a material being held for an excessive amount of time in the precipitation temperature range. [16]
The table 1 below shows the composition of stainless steel 304.
| Grade | carbon | manganese | silicon | phosphorous | sulfur | chromium | nickel | nitrogen | |
| 304 | Min | – | – | – | – | – | 18.0 | 8.0 | – |
| Max | 0.08 | 2.0 | 0.75 | 0.045 | 0.03 | 20.0 | 10.5 | 0.10 |
Stainless Steel 304 offers a number of advantages:
Rust proof and high corrosion resistance – The 304 grade of stainless steel has a very good resistance to corrosion in various types of environments. It does not rust in most of the architectural applications. It is resistant to atmospheric corrosion and also in an environment where there is food being processed, the stainless 304 is resistant and unaffected. It can be cleaned easily and does not react with organic and inorganic chemicals in an environment which is either oxidizing or reducing. Dyes or other types of chemicals do not effect it as well. The stainless steel 304 alloy also has a high chromium content present which makes it resistant to oxidizing chemicals like nitric acid up to 55 % weight and up to 80 degrees Celsius. It also does not react to organic acids that are commonly aggressive such as acetic. The nickel that is present in the alloy provides resistance to solutions that are commonly reducing such as phosphoric acid of any concentration in solutions that are cold and up to 10 percent dilute in solutions that are hot. In fresh water if there are levels of chlorides present then this alloy has a good performance but in an environment of warn chloride, stainless steel 304 can be subject to crevice and pitting corrosion and also to stress corrosion cracking when it is subject to increased tensile stresses after about 50 degrees Celsius. When the exposure is not continuous in these types of environments and it is cleaned on a constant basis then then stainless steel 304 is not affected. [18, 19]
For sure! Our writers with advanced degrees dive into any topic—think quantum physics or medieval lit—with deep research and clear, sharp writing. They’ll tailor it to your academic level, ensuring it’s thorough yet easy to follow, no matter how tricky the subject gets.
Recyclable – Stain steel is a 100 percent recyclable. When the product has been used and its service life has ended then the alloys that are present in it that are chromium, nickel and molybdenum can be easily retrieved from the alloy. They can then be returned to the production process. The recycling process of stainless steel is self-sustaining and is also environmentally feasible. The production methods which use recyclable materials have an impact on the savings in energy and also become the reason of decreased carbon dioxide emissions which is substantial. In a stainless steel product, 60 percent of it contains recycled materials. When the scrap metal is recycled there is scrap metal that is generated which is also recycled in the same way. The scrap that is to be used is limited but as the use of stainless steel is growing therefore this percentage will increase. [20]
 Antibacterial properties- It has good antibacterial properties and it does not stain so it can be cleaned and re-used many times in the medical field. Stainless steel does not have any antimicrobial effect that is active but it is still the type of material widely used in medical application and other applications that require hygiene. This is due to the reason that it can be cleaned and sanitized easily even after being used for a very long time in comparison to the other metals of which the performance will change. Stainless steel is also inert therefore it does not cause the microorganisms to form any type of resistance. Other surfaces which are active when compared to stainless steel, release a lot of metal ions into the environment of which the effects are not fully known. Stainless steel can be sterilized in many ways while other metals can have coatings which might have reactive nature which limits there options. [21]
Antibacterial properties- It has good antibacterial properties and it does not stain so it can be cleaned and re-used many times in the medical field. Stainless steel does not have any antimicrobial effect that is active but it is still the type of material widely used in medical application and other applications that require hygiene. This is due to the reason that it can be cleaned and sanitized easily even after being used for a very long time in comparison to the other metals of which the performance will change. Stainless steel is also inert therefore it does not cause the microorganisms to form any type of resistance. Other surfaces which are active when compared to stainless steel, release a lot of metal ions into the environment of which the effects are not fully known. Stainless steel can be sterilized in many ways while other metals can have coatings which might have reactive nature which limits there options. [21]
Stainless steel is also used in a lot of home and commercial applications. It is the most common and frequently used type of alloy in comparison to other stainless steel alloys. Its applications include containers and also tanks for different types of solids and liquids. In the food industry the food processing equipment used for the brewing of beer, making wine and the processing of milk is made of this alloy as it is suitable. It can applied in dairy equipment such as milking machines, containers, homogenizers, sterilizers, and storage and hauling tanks, including piping, valves and milk trucks. In the brewing industry it is used in pipelines, yeast pans, fermentation vats and storage. The fruit industry also uses it for handling, crushing, preparation, storage and hauling equipment. As stainless steel 304 has the ability to resist corrosion of different types of acids found in fruits, meats, milk and vegetables, it is used for sinks, tabletops, coffee urns, stoves, refrigerators, milk and cream dispensers, and steam tables. It is also used in many other appliances such as pots, cooking appliances, flatware and pans. There are other materials that stainless steel 304 used to make such as architectural paneling, railing and trims. Containers that are used to hold chemicals including the ones used in transporting them. Heat exchangers, screens that are welded for mining, quarrying and the filtration of water. In a marine environment, it is used to make nuts, bolts, screws and also other fasteners due to its higher strength and resistance to wear. [22]
Stainless steel of grade 316L is the lower carbon version of the 316 grade stainless steel. It has a higher percentage of nickel alloy then the stainless steel grade 304 and also contains molybdenum alloy. The composition this grade of stainless steel has makes its corrosion resistance properties better than that of the stainless steel grade 304 in many environments that are aggressive. The table below shows the composition of stainless steel 316L.
We stick to your rubric like glue—nailing the structure, depth, and tone your professor wants—then polish it with edits for that extra shine. Our writers know what profs look for, and we double-check every detail to make sure it’s submission-ready and grade-worthy.
| Grade | carbon | manganese | silicon | phosphorous | sulfur | chromium | molybdenum | nickel | nitrogen | |
| 316L | Min | – | – | – | – | – | 16.0 | 2.00 | 10.0 | – |
| Max | 0.03 | 2.0 | 0.75 | 0.045 | 0.03 | 18.0 | 3.00 | 14.0 | 0.10 |
The addition of the molybdenum makes the grade 316L more resistant to pitting and crevice corrosion in environments which have chlorides and also sea water. It also makes it also very effective in an environment which is acidic and it protects the metal against the corrosion caused by sulfuric, hydrochloric, acetic, formic and tartaric acids and also acid sulfates and alkaline chlorides. The stainless steel grade 316L is also not affected by sensitization, which is the precipitation of the carbides from the grain boundaries, as is has lower carbon. It is an austenite type of steel due to which it has high toughness even if it is being used in cryogenic temperatures. The most common types of uses for this type of stainless steel grade is in the construction of manifolds for exhausts, parts for the furnaces, heat exchangers, parts for jet engines, photographic and pharmaceutical equipment, pump and valve parts, equipment’s required for the processing of chemicals, tanks, evaporators, textile and papers processing equipment and also parts that are going to be exposed to a marine environment. [23, 24, 25]
Stainless steels have excellent corrosion resistance due to which they are used excessively in the alimentary industry and also in the medical industry. They are used mostly for implants, medical devices and controlled drug delivery systems production. The most common type of stainless steel type used in medicine is 316L. The stainless steel grade 316L does not have reactions that are toxic with the surrounding tissues and they are also used extensively in devices to be used in the surgical treatment of injuries such as fracture plates, screws and hip nails along with other devices due to their low cost, accessibility and also easy processing. The austenite types of stainless steels have a good combination of mechanical, fabrication and corrosion resistance properties but the austenite types of stainless steels are reactive to some corrosive environments to corrosive attacks such as pitting corrosion in which cavities are produced in the material and inter crystalline corrosion which happens when the chromium carbides start precipitating from the grain boundaries at high temperatures. The stainless steel type 316L has a very high corrosion resistance due to the chromium content that it contains.
A passive layer is formed on the surface stainless steel 316L due to the chromium content. It also has low carbon due to which chromium carbides are prevented from being formed in the grain boundaries. This also decreases the chances of material to have intergranular corrosion. Pitting corrosion in an environment of chlorides is also prevented due to the alloy of molybdenum while the structure which is austenite is also stabilized due to the alloy of nickel. The stainless steel type 316L can be welded without the need of it being heat treated. In the case of medical applications, the decontamination and also the sterilization of the medical devices is also required. [27].
Send us your draft and tell us your goals—we’ll refine it, tightening arguments and boosting clarity while keeping your unique voice intact. Our editors work fast, delivering pro-level results that make your paper pop, whether it’s a light touch-up or a deeper rework.
Even though the austenite type of stainless steel 316L is more resistant to stress corrosion cracking in comparison with the other types of stainless steel due to the molybdenum content that is present in the 316L type of stainless steel, it may still be vulnerable to stress corrosion cracking. Some of the conditions which may cause this type of corrosion to occur are: presence of halide ions, residual tensile stresses, and also temperatures that might be more than 49 degrees Celsius. Stress may occur from cold deformation or the thermal cycles when this steel is being welded. These stresses can be reduced by annealing or stress relieving heat treatments which will also then reduce the sensitivity to halide stress corrosion cracking. Even though the stainless steel type 316L does not offer an advantage with regards to stress corrosion cracking, they are still better to use in the stress relieved conditions in the types of environments which may cause intergranular corrosion. [28]
The stainless steel grade 316 is vulnerable to intergranular corrosion which is the precipitation of the chromium carbides in the grain boundaries. This takes place when this grade of stainless steel is exposed to temperatures consisting of a range between 427 degrees Celsius and 816 degrees Celsius. These steels which are in a sensitized state will be effected by intergranular corrosion when exposed to environments which are aggressive. Due to this reason the lower carbon version of stainless steel grade 316 which is 316L is used when available to avoid the danger of intergranular corrosion. These stainless steels grade 316L provide resistance from intergranular corrosion with any thickness in the welded condition or even when exposed for a short time to temperatures ranging from 427 degrees Celsius to 816 degrees Celsius. When the materials require stress relieving treatments, short treatments that fall within this range of temperatures can be done without effecting the corrosion resistance the metal normally has. For the lower carbon grades, fast cooling from the high temperatures is not required when the bulky or heavy sections of the metal have been annealed. This grade of stainless steel has the same advantageous corrosion resistance and mechanical properties such as those of the higher carbon version of this stainless steel and also offer the additional benefit in applications that are highly corrosive where there are chances of intergranular corrosion taking place. Even though exposure to high temperatures during welding or stress relieving will not cause intergranular corrosion to take place, the fact that a long time of exposure to high temperature will be harmful for the stainless steel grade 316L should be taken into regard. Stress relieving this stainless steel between 593 degrees Celsius and 816 degrees Celsius will cause the metal to have a loss of ductility. [28]
This stainless steel grade 316L also shows great resistance to oxidation and also had a low rate of scaling in air atmospheres at a temperature range between 871 degrees Celsius and 899 degrees Celsius. As the rate of oxidation is greatly affected by the type of atmosphere it is present in and the conditions of operations that are being used, no specific data can be depicted where all the conditions are there. [28]
The austenite type of stainless steel is not magnetic in the annealed fully austenite condition. When it is annealed, the magnetic permeability of the stainless steel grade 316L is less than 1.02 oersteds at 200 H. The values of permeability vary for the material that is cold deformed with the composition and also the amount of cold deformation. They are usually higher in comparison to that of an annealed material. [28]
The type of metal that is going to be used depends on the specific application of the implant. The stainless steel type 316L is the most used alloy in all the implant divisions ranging from cardiovascular which relates to the heart and blood vessels and to otorhinolaryngology which deals with the states related to those of the ear nose and the throat and also the head and the neck. For an extensive time period metals had been used for implants but the problem with them was that they corroded and they were not able to handle the amount of load. When this stainless steel grade was discovered, it a lot of clinicians were interested in it as at that time it was the most corrosion resistant metal in comparison with others. . It is used in many biomedical divisions. In the cardiovascular division it is used to make the stent which a small mesh tube that’s used to treat narrow or weak arteries. It is also used to make the artificial valve of the heart. In the orthopaedic division, the plate, screws and pins for bone fixation and also the artificial joints are made using stainless steel 316L. In the dentistry division, it is used to make the orthodontic wire which is used with the braces to align the teeth. Fillings are also made using this stainless steel 316L. The plate and screw in the craniofacial division is made using stainless steel 316L. The artificial eardrum is also made using stainless steel 316L. [29]
Yes—we’ve got your back! We’ll brainstorm fresh, workable ideas tailored to your assignment, picking ones that spark interest and fit the scope. You choose the winner, and we’ll turn it into a standout paper that’s all yours.
All the implants made of this stainless steel grade 316L are non-magnetic and have a high density. This is very important as the implants need to compatible with the magnetic resonance imaging and also need to be visible under X-ray imaging. A lot of these metals are to be implanted will be subjected to loads that might be static or repetitive and therefore it is a condition that must be met that the metal should have good strength and ductility. These are the types of characteristics that makes stainless steel 316L superior then polymers and ceramics. Stents and the stent grafts are implanted in the body to open blocked blood vessels therefore it is required that the plasticity for expansion and rigidity to maintain the action of dilating the vessel. For the orthopaedic implants, the metals must have good elasticity, toughness, rigidity, strength and resistance to fracture. If the joint is going to be replaced completely than then the metal needs to be able to wear resistance so that the debris formation from the friction can be avoided. Dentists require the materials to be used to be strong and rigid. [29]
The process of heat treatment which the material will go through depends on the type of stainless steel that is to be heat treated and the reason that it going to be treated for. Various types of the heat treatment methods effect the stainless steel in different ways. Stress relieving, hardening and annealing increase the strength of ductility and also the resistance to corrosion properties of the stainless steel that is going to be effected while being fabricated. The heat treatment of the various stainless steel should be carried out under certain conditions to make sure that metal surface does not carburize, decarburize and scaling doesn’t occur. [41]
Before the heat treatment process is to be performed on the stainless steel, the surface of the stainless steel has to be thoroughly cleaned. This is done to remove any residues such as that of carbon, grease and oil. These residues might cause carburization to occur and this will reduce the corrosion resistance properties of the stainless steel. [41]
There are a lot of methods in which the stainless steel can be annealed. The austenite type of stainless steel cannot be hardened by being heat treated but they be hardened by being cold worked. Annealing the stainless steel causes the recrystallization of the grains that were work hardened (toughening the material by cold working). It also causes the chromium carbides to be placed back into the solution. Anneal can also be used to relieve the stresses caused by cold working of the material. To anneal stainless steel, temperatures above 1040 degrees Celsius are required. [42]
Annealing of the austenite type of stainless steel is known as quench annealing as the metal has to be cooled rapidly when it has the temperatures ranging from 1040 degrees Celsius to those below 600 degrees Celsius. This is to avoid sensitization which is the precipitation of carbides at the grain boundaries. [42]
The hardness of the martensitic type of stainless steels is increased by austenitizing, quenching and tempering. The temperatures of austenitizing them has a range between 980 degrees Celsius and 1010 degree Celsius. The hardness of the quenched stainless steel increase up to a temperature of 980 degrees Celsius and then it decrease because of the presence of retained austenite. A slow heating rate before the austenization process is to be used to prevent cracks from forming in stainless steels that have high carbon content. When the stainless steel reaches the austenite temperature, it is cooled using air so that is forms full hardness. When the stainless steel reaches room temperature, it should be immediately tempered to avoid cracking. [42]
To stress relieve the stainless steel, it his heated between 425 degrees Celsius and 925 degrees Celsius. Heating the stainless steel at 870 degrees Celsius relieves the material from 85 percent of the stress. When the stainless steel is stress relieved at this temperature range, sensitization will occur which is the precipitation of chromium carbides from the grain boundaries. This decrease the corrosion resistance of the stainless steel. [42]
Yep—need changes fast? We’ll jump on your paper and polish it up in hours, fixing whatever needs tweaking so it’s ready to submit with zero stress. Just let us know what’s off, and we’ll make it right, pronto.
Stress relieving the stainless steel decreases the residual stress and also causes the stainless steel to avoid stress corrosion cracking. It also increase the notch sensitivity of the stainless steel which is the ability which that material has to absorb the energy in the presence of a flaw. It also improves the dimensional stability, which is the amount of which the material retains its original dimensions when it is exposed to change in temperature and humidity, of the stainless steel in use. During fabrication, a large amount of stainless steel is welded. When it not possible for some components to be fully annealed, the weldments can be heated to a medium temperature to decrease the high residual stresses. [42]
When the austenite type of stainless steel has been cold worked to increase the amount of strength it has, stress relieving it at a low temperature in a range between 160 degrees Celsius to 415 degrees Celsius will decrease the residual stresses without effect on the corrosion resistance or the mechanical properties of the stainless steel. If the resistance to intergranular corrosion is not required then temperatures up to 425 degrees Celsius can be used. [42]
If ductility is required then the stainless steel can be tempered. Steel that is not tempered is very hard but it cannot be used for many applications as it is too brittle. Tempering is a process in which a temperature that is not too high is used and is usually performed after the hardening of the steel in order to reach the ratio of hardness to toughness that is required. In this process, the metal is heated to a low temperature which reduces some of the hardness. After this, the metal is cooled using air which results in the metal being tougher and less brittle. [43]
A study was carried out by S.A. Tukur, M.S Dambatta, A. Ahmed and N.M. Mu’az to find the effect of heat treatment temperature on the mechanical properties of stainless steel 304. Samples of stainless steel 304 were obtained from the quality and assurance mechanical laboratory of the Delta steel company in Nigeria and the sample had a composition consisting of 0.08% carbon, 18.75 percent Chromium, 1 percent Silicon, 10.5 percent Nickel and 2 percent Manganese. [44]
A total of 12 sample were sensitized in a high temperature furnace at a temperature of 660 degrees Celsius for a period of 30 minutes. The samples were then in an open air environment. These samples were then solution annealed at different temperatures. [44]
Based on the international standards, parts were machined from the treated stainless steel 304 samples. Mechanical tests were then conducted on these samples to find out there tensile and hardness properties. To find out the hardness of the samples, HV-1000 Rockwell type digital micro hardness testing machine was used. The value was obtained by taking the average of five values at different positions of the same sample. The tensile test was also conducted on the samples by using a 600 KN Avery-Denison universal test machine. The ends of the samples were gripped in the machine and a load was applied until the sample failed. [44]
Sure thing! We’ll whip up a clear outline to map out your paper’s flow—key points, structure, all of it—so you can sign off before we dive in. It’s a handy way to keep everything aligned with your vision from the start.
Figure six on the right shows the micro hardness of the samples annealed at different temperatures. The sample that was sensitized at a temperature of 660 degree Celsius had the highest hardness value compared to the samples at other temperatures. This increase in the value of Hardness could be due to the precipitation of chromium carbides at the grain boundaries. The precipitated chromium carbides stop the dislocation movement and reduce the defects that are in the crystals lattice of the sample that is sensitized. The movement of the dislocations is usually referred as the dominant carrier of the plasticity which is the quality of it being easily shaped. There was a sharp decrease in the Hardness value of the samples between the temperature of 1010 degrees Celsius and 1050 degrees Celsius. There is a small increase in the Hardness at a temperature of 1090 degrees Celsius. The decrease is due to the dissolution of the carbide and the chromium enrichment in the matrix while the increase could be due to the sample matrix being stretched at that temperature thus increasing the strength of the sample. As it also preferred by the carbides to be homogenously in the solution before the beginning of the cooling stage. The chromium carbides dissolve slowly. Due to these reasons, the highest temperature in which the stainless steel can be heated with a limited grain growth in this case is 1090 degrees Celsius. The samples heat treated above these temperatures have a decrease in the hardness of the samples which can be due to the increase in the size of the grain. The results also showed that the samples annealed at higher temperatures had ridden themselves of the formed alloy segregation and sigma phase which is an intermetallic phase that is made of iron and chromium which is non-magnetic and forms in austenite stainless steels when exposed to temperatures ranging from 560 degrees Celsius to 980 degree Celsius. This resulted in an increase in the ductility of the stainless steel samples at the higher temperatures. Dissolution of chromium carbides at the grain boundaries was also the reason as it stopped the dislocation movement. [44]
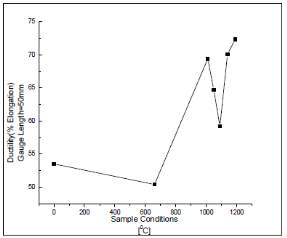 The figure on the right compared the results of the yield strength and the ultimate tensile strength that were found of the samples in this study. It shows that the sample sensitized at 660 degrees Celsius had highest strength compared to the untreated sample as well as the samples annealed at higher temperatures. This could be due to the formation of chromium carbides in the grain boundaries of the sample which restrict the movement of the dislocations hence causing an increase in the yield strength of the materials. The temperatures that were annealed at higher temperatures had a sharp decrease in the strength of the samples which could be due to the dissolution of the carbide and the chromium enriched areas in the matrix of the sample thus causing a decrease in the strength of the sample. It was observed that there was a slight increase after 1000 degrees Celsius which could be due to the grain growth in the microstructure of the samples. [44]
The figure on the right compared the results of the yield strength and the ultimate tensile strength that were found of the samples in this study. It shows that the sample sensitized at 660 degrees Celsius had highest strength compared to the untreated sample as well as the samples annealed at higher temperatures. This could be due to the formation of chromium carbides in the grain boundaries of the sample which restrict the movement of the dislocations hence causing an increase in the yield strength of the materials. The temperatures that were annealed at higher temperatures had a sharp decrease in the strength of the samples which could be due to the dissolution of the carbide and the chromium enriched areas in the matrix of the sample thus causing a decrease in the strength of the sample. It was observed that there was a slight increase after 1000 degrees Celsius which could be due to the grain growth in the microstructure of the samples. [44]
The figure 8 on the right shows the ductility of the samples that were measured against the temperatures they were heat treated at. The ductility decreases of the sample that is sensitized at a temperature of 660 degrees Celsius. This could be due to the reason that the movement of the dislocations being hindered by the precipitated chromium carbides. There is an increase in the ductility after which there is a decrease and then an increase again. The increased ductility is because of the solution annealing treatment at high temperatures causes an increase in the number of planes on the samples that were treated where the dislocation movement can occur. [44]
Figure 8 variation of the ductility of the samples with the temperature they were heat treated to
Absolutely—we’ll weave in sharp analysis or eye-catching visuals like stats and charts to level up your paper. Whether it’s crunching numbers or designing a graph, our writers make it professional and impactful, tailored to your topic.
In conclusion, in this study the effect of the heat treatment process and the temperature the sample is to be heated to was assessed. It was found that the highest hardness value of the sensitized sample was that at 660 degrees Celsius while for the solution annealed sample it was at 1090 degrees Celsius. This was the temperature they found to be the best to avoid the growth of grains in the solution annealed stainless steel sample. [44]
In a study done by P. Atanda, A. Fatudimu and O. Oluwole different samples of stainless steel type 316L were sensitized to find out how it affected the microstructures and the hardness of the samples. There were a total of 20 samples. Each four samples were heat treated to the same temperature but for a different amount of time. This can be seen in the table below. [45]
Table 3 the different temperatures each sample was heat treated to
| 750 degrees Celsius | 30 minutes |
| 1 hour | |
| 2 hours | |
| 8 hours | |
| 800 degrees Celsius | 30 minutes |
| 1 hour | |
| 2 hours | |
| 8 hours | |
| 850 degrees Celsius | 30 minutes |
| 1 hour | |
| 2 hours | |
| 8 hours | |
| 900 degrees Celsius | 30 minutes |
| 1 hour | |
| 2 hours | |
| 8 hours | |
| 950 degrees Celsius | 30 minutes |
| 1 hours | |
| 2 hours | |
| 8 hours |
Before the samples were heat treated, each of the sample was grinded on silicon carbide paper. While being grinded, the surface of the paper was continuously being flushed with water to act as a lubricant and also to wash away any excess particles that could have damaged the samples. The grades that were used to grind the samples were 120, 240, 320 and 400 grits in the same order as mentioned. [45]
After the grinding process, the samples were polished on a polishing cloth using alumina powder which had been dissolved in water at a suitable proportion. The powder that was used was of the grade 1 micron and 0.3 micron. Pressure was applied until the sample were free of any scratches. The samples were then cleaned and dried after which they were examined using a microscope with a magnification between 50 and 100. This was to make sure that the samples were free of any scratches. [45]
After the samples had been polished, they were etched in a solution made of 5gm of Iron (III) chloride + 10ml of Hydrochloric acid + 50ml of water. To etch the samples, they were washed to remove any polishing compound that had been left on it and then they were put into the etching solution for a total of three minutes. The samples were then transferred to running water to wash away the etchant and it was seen to what extent the sample had been etched. The samples that had etched successfully appeared dull. After this, the samples were observed using an optical microscope and the photomicrographs were taken. [45]
Results
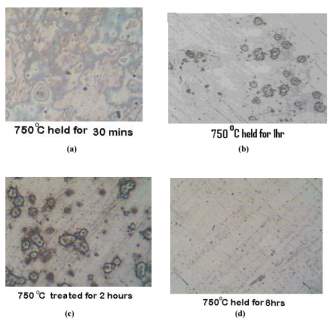 The photomicrographs of the samples that were normalized at temperature of 750 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 9 on the right. [45]
The photomicrographs of the samples that were normalized at temperature of 750 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 9 on the right. [45]
Chromium depleted zones can be seen here but are negligible in the sample held for 8 hours at 750 degrees eight hours. [45]
We tackle each chunk with precision, keeping quality consistent and deadlines on track from start to finish. Whether it’s a dissertation or a multi-part essay, we stay in sync with you, delivering top-notch work every step of the way.
The photomicrographs of the samples that were normalized at temperature of 800 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 10 on the right. [45]
Chromium depleted zones can be also be seen here and are slightly clear in the sample held for 8 hours at 800 degrees eight hours. [45]
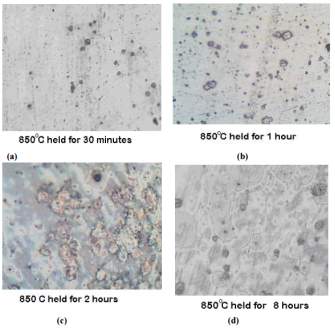 The photomicrographs of the samples that were normalized at temperature of 850 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 11 on the right. [45]
The photomicrographs of the samples that were normalized at temperature of 850 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 11 on the right. [45]
Yes—we’ve got it down! Our writers switch seamlessly between UK, US, Australian, or any other standards, matching your school’s exact expectations. Your paper will feel native to your system, polished and ready for wherever you’re studying.
Chromium depleted zones can be also be seen here and but are well formed for the sample treated for 8 hours at 850 degrees as compared to those samples that were treated at a lower temperature. [45]
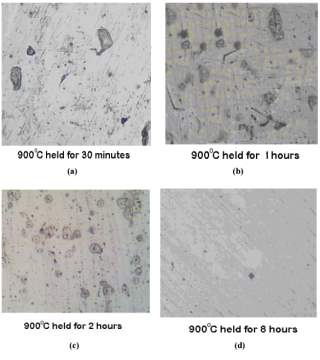 The photomicrographs of the samples that were normalized at temperature of 900 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 11 on the right. [45]
The photomicrographs of the samples that were normalized at temperature of 900 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 11 on the right. [45]
Chromium depleted zones can only be seen in the samples treated for 1 and 2 hours respectively but are absent in the samples that were treated for 30 minutes and 8 hours. [45]
Progressive delivery is a cool option where we send your paper in chunks—perfect for big projects like theses or dissertations. You can even pay for it in installments. It’s just 10% extra on your order price, but the perks are worth it. You’ll stay in closer touch with your writer and can give feedback on each part before they move to the next. That way, you’re in the driver’s seat, making sure everything lines up with what you need. It saves time too—your writer can tweak things based on your notes without having to redo huge sections later.
The photomicrographs of the samples that were normalized at temperature of 950 degrees Celsius held for the period of 30 minutes, 1 hour, 2 hours and 8 hours can be seen in figure 11 on the right. [45]
Chromium depleted zones are negligible but can be seen faintly in the samples treated for 1 and 2 hours respectively. [45]
Absolutely! If your teacher’s got feedback, you can request a free revision within 7 days of approving your paper—just hit the revision request button on your personal order page. Want a different writer to take a crack at it? You can ask for that too, though we might need an extra 12 hours to line someone up. After that 7-day window, free revisions wrap up, but you can still go for a paid minor or major revision (details are on your order page). What if I’m not satisfied with my order? If your paper needs some tweaks, you’ve got that free 7-day revision window after approval—just use the “Revision” button on your page. Once those 7 days are up, paid revision options kick in, and the cost depends on how much needs fixing. Chat with our support team to figure out the best way forward. If you feel the writer missed the mark on your instructions and the quality’s off, let us know—we’ll dig in and sort it out. If revisions don’t cut it, you can ask for a refund. Our dispute team will look into it and figure out what we can offer. Check out our money-back guarantee page for the full scoop.
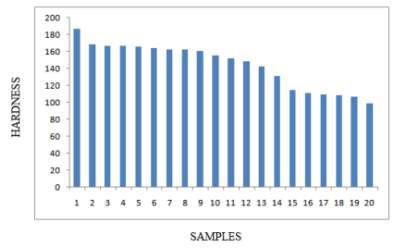
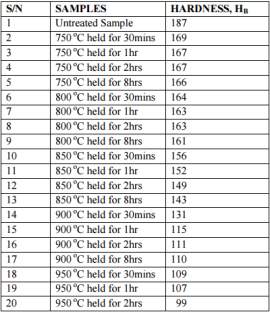 In this study it was observed that there was a considerable difference in the photo microstructures of the heat treated samples in relation to the amount of time the samples were heat treated to. The chromium depleted zones increased considerably for the sample treated upto 2 hours while they decreased in the samples treated for 8 hours. The table 4 on the right and figure 14 below shows the Brinell hardness values of the samples that were heat treated. It can be clearly seen that the value of the Brinell hardness decrease as the temperature increases and the time it is heat treated for is increased. [45]
In this study it was observed that there was a considerable difference in the photo microstructures of the heat treated samples in relation to the amount of time the samples were heat treated to. The chromium depleted zones increased considerably for the sample treated upto 2 hours while they decreased in the samples treated for 8 hours. The table 4 on the right and figure 14 below shows the Brinell hardness values of the samples that were heat treated. It can be clearly seen that the value of the Brinell hardness decrease as the temperature increases and the time it is heat treated for is increased. [45]
 It can be seen in the microstructures that the black patches form the depleted zone. The width of these zones increase with the increase in the time which leads to a flatter chromium profile close to the grain boundaries for the temperatures between 750 and 850 degrees Celsius and for a short time at these two temperatures between 30 minutes and 2 hours. The process of heat treatment cause significant changes in the chromium concentration profile. The process in which the samples were heat treated for a shorter time caused sensitization to occur this breakdown in the corrosion resistance occurs in austenite stainless steels when they are heat treated in a temperature range between 550 and 850 degrees Celsius. During carbide precipitation, the interstitial carbon diffuses quickly to the grain boundaries. The chromium diffusion rate is much slower which results in chromium depleted zones at the grain boundaries. In this stage the stainless steel is susceptible to intergranular corrosion. After a long time of heat treatment at higher temperatures, the desensitization started to occur when the chromium content at the carbide matrix increased due to the chromium diffusion from the matrix further away from the grain boundary. This is the reason due to which it did not have any harmful effect on the stainless steel 316L sample. [45]
It can be seen in the microstructures that the black patches form the depleted zone. The width of these zones increase with the increase in the time which leads to a flatter chromium profile close to the grain boundaries for the temperatures between 750 and 850 degrees Celsius and for a short time at these two temperatures between 30 minutes and 2 hours. The process of heat treatment cause significant changes in the chromium concentration profile. The process in which the samples were heat treated for a shorter time caused sensitization to occur this breakdown in the corrosion resistance occurs in austenite stainless steels when they are heat treated in a temperature range between 550 and 850 degrees Celsius. During carbide precipitation, the interstitial carbon diffuses quickly to the grain boundaries. The chromium diffusion rate is much slower which results in chromium depleted zones at the grain boundaries. In this stage the stainless steel is susceptible to intergranular corrosion. After a long time of heat treatment at higher temperatures, the desensitization started to occur when the chromium content at the carbide matrix increased due to the chromium diffusion from the matrix further away from the grain boundary. This is the reason due to which it did not have any harmful effect on the stainless steel 316L sample. [45]
In conclusion it was observed in this study that the stainless steel samples began to sensitize when heated to temperatures ranging from 750 degrees Celsius and 850 degrees Celsius while being held for time range from 30 minutes to 2 hours before normalizing. When the time was increased for soaking, the desensitization process had finished completely in the sample heat treated at 750 degrees Celsius but continued for the samples at 800 degrees Celsius and 850 degree Celsius. At a higher temperature of 900 degrees Celsius sensitization occurred when soaked for 1 and 2 hours before normalizing. At a longer time of soaking for 8 hours before normalization, the sample desensitized completely. For the sample that was heat treated at 950 degrees Celsius sensitization occurred for the sample soaked for 30 minutes and desensitization occurred for the samples soaked for 1 and 2 hours. For the sample soaked for 8 hours, complete desensitization had occurred. Thus it was observed that for the sample at 950 degrees Celsius, the chromium diffusion was being aided thermally making it faster and cancelling out the initial sensitization. It was seen that the Hardness of the stainless steel 316L samples also decreased with the increase in soaking time as well as the normalization temperature. [45]
For the testing phase, three samples of stainless steel grade 304 will be heated to three different temperatures. The three samples are going to be placed in a heat furnace. Each sample will be put separately as each of the sample will be heated to a different temperature. The temperatures each of the samples will be heated to will be 300, 400 and 800 degrees Celsius.
| Metal | Stainless steel grade 304 | ||
| Sample | 1 | 2 | 3 |
| Temperature (degrees C) | 300 | 400 | 800 |
 As these samples will be heated, there will be a change in the properties of the microstructures and also in the macrostructure. Changes occur to the microstructures of stainless steel when they are exposed to high temperatures for a period of time. All the metal samples will be looked through a microscope before and after the test to observer the changes that have taken place and pictures will be taken of the microstructures. All of these will then be compared to see which temperature brought what type of difference and how the microstructures of that specific metal changed to comparison to the other sample which was not heat treated and was at room temperatures. The stainless steel 304 samples will also be tested to check there mechanical properties, which will be compared to the mechanical properties that sample has when it is at room temperature. There will be a difference in the properties of each of the different samples at three different temperatures. These will also be compared to that of their room temperatures.
As these samples will be heated, there will be a change in the properties of the microstructures and also in the macrostructure. Changes occur to the microstructures of stainless steel when they are exposed to high temperatures for a period of time. All the metal samples will be looked through a microscope before and after the test to observer the changes that have taken place and pictures will be taken of the microstructures. All of these will then be compared to see which temperature brought what type of difference and how the microstructures of that specific metal changed to comparison to the other sample which was not heat treated and was at room temperatures. The stainless steel 304 samples will also be tested to check there mechanical properties, which will be compared to the mechanical properties that sample has when it is at room temperature. There will be a difference in the properties of each of the different samples at three different temperatures. These will also be compared to that of their room temperatures.
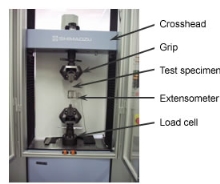
To find the properties of the metals, different types of tests will be conducted. These tests will be done to find the corrosion resistance of the sample when it is placed in a liquid known as etching solution and the hardness of the metals using the Vickers hardness test machine. The tensile strength, yield strength and the elongation will be found by doing the tensile test on the specific type of stainless steel 304 sample. To perform these tests, the metal will be placed in the  machine and will be pulled. After this is done, it will be determined how the material reacts to the forces applied when in tension and the strength will be found by observing its elongation. The yield strength will be the value of the metal when the stress applied to the material while it is being pulled will cause plastic deformation to occur. The Hardness test will determine the hardness of the samples which is the property of that metal to lets it to resist plastic deformation. The Vickers Hardness test will be done to determine the hardness of the samples.
machine and will be pulled. After this is done, it will be determined how the material reacts to the forces applied when in tension and the strength will be found by observing its elongation. The yield strength will be the value of the metal when the stress applied to the material while it is being pulled will cause plastic deformation to occur. The Hardness test will determine the hardness of the samples which is the property of that metal to lets it to resist plastic deformation. The Vickers Hardness test will be done to determine the hardness of the samples.
The ultimate tensile strength is also one of the properties that will be determined of all the samples. This is the highest load that the samples will be able to withstand when they are being tested. This value will depend on the type of sample including the heat treatment that it had gone through and the specific properties that metal had initially.
 As heat treatment will be done using the heat furnace at a high temperature, care will have to be taken. It will be made sure that there is no material that is combustible near the furnace as it will cause a risk of fire to occur. Ventilation should also be proper as the high temperatures can pose danger if they are not handled carefully. Gloves and goggles will be worn.
As heat treatment will be done using the heat furnace at a high temperature, care will have to be taken. It will be made sure that there is no material that is combustible near the furnace as it will cause a risk of fire to occur. Ventilation should also be proper as the high temperatures can pose danger if they are not handled carefully. Gloves and goggles will be worn.
The contact angles will also be found to assess the wettability of the stainless steel 304 samples. The contact angle is the angle which the liquid creates with the surface of the solid when they both come into contact together.
Before the samples were tested, they had to go through some treatment phases. After this treatment the micro hardness test could be carried out on the samples. Below are steps that took place initially and it can be seen which of the following phases the samples had gone through before testing the samples.
The actual testing that had taken place was very similar to that which had been designed but only with some slight changes such as the number of samples tested. There were a total of 9 samples of which 8 samples were heat treated to different temperatures as shown in the table below.
Table 6 Temperature samples were heat treated to
| # | Sample 0 | Sample |
You Want The Best Grades and That’s What We Deliver
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
We offer the lowest possible pricing for each research paper while still providing the best writers;no compromise on quality. Our costs are fair and reasonable to college students compared to other custom writing services.
You’ll never get a paper from us with plagiarism or that robotic AI feel. We carefully research, write, cite and check every final draft before sending it your way.