Top Essay Writers
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
Simply fill out the order form with your paper’s instructions in a few easy steps. This quick process ensures you’ll be matched with an expert writer who
Can meet your papers' specific grading rubric needs. Find the best write my essay assistance for your assignments- Affordable, plagiarism-free, and on time!
Posted: January 2nd, 2024
THE INFLUENCE OF DIFFERENT TIME (MIN) AND HEATING TEMPERATUES (°C) OF WATER ON VITAMIN C AND SUGAR ABUNDANCE IN RED DELICIOUS APPLES (100G)
ABSTRACT
Students often ask, “Can you write my essay in APA or MLA?”—and the answer’s a big yes! Our writers are experts in every style imaginable: APA, MLA, Chicago, Harvard, you name it. Just tell us what you need, and we’ll deliver a perfectly formatted paper that matches your requirements, hassle-free.
Fruits as we all know are crucial to maintaining a healthy body and the most common fruits are apples; as it provides many nutrients and antioxidants. One of the main nutrients that apples contain is Vitamin C. It is one of the most effective antioxidants, which help to grow and repair tissues in all parts of our bodies, for example healing a cut wound. Without enough Vitamin C present in our body, if a cut was present then harmful chemicals and substances within the atmosphere will be able to travel through our bloodstream. Also, there is another important but harmful when overdosed nutrient which is fructose and glucose. As a person who hates crunchy food, and is already overdosing with sugar levels, it led me to the question:
“To what extent does the different temperature of water (50°C, 70°C and 100°C) and time (3min and 6min) that the apple (Red Delicious) is exposed to, affect the Vitamin C (ascorbic acid) and sugar content in Red Delicious, by measuring the concentration of Vitamin C and sugar still remaining in g per 100g of mass?” (e.g. exposing the apple in 50°C for 3 and 6min. Then exposing the apple in 70°C for 3min and 6min etc.)
The 2 independent variables can be measured due to comparing the results of different temperatures at the same time (either 3min or 6min), or different time but same temperature.
For vitamin C content it was done through iodine titration, and for sugar content, the density of the apple sample was compared to the graph of standardized sugar solution (density vs. amount of sugar).
Absolutely, it’s 100% legal! Our service provides sample essays and papers to guide your own work—think of it as a study tool. Used responsibly, it’s a legit way to improve your skills, understand tough topics, and boost your grades, all while staying within academic rules.
This ultimately lead me to wonder, is there a correlation between Vitamin C and sugar content and how it is affected with an increase in temperature and time; thus, determining just how much sugar is within 1 mg of vitamin C by finding out the ratio, and the findings were phenomenon.
WORD COUNT: 318
CONTENT:
Our pricing starts at $10 per page for undergrad work, $16 for bachelor-level, and $21 for advanced stuff. Urgency and extras like top writers or plagiarism reports tweak the cost—deadlines range from 14 days to 3 hours. Order early for the best rates, and enjoy discounts on big orders: 5% off over $500, 10% over $1,000!
CHAPTER 1
CHAPTER 2
CHAPTER 3
CHAPTER 4
Yes, totally! We lock down your info with top-notch encryption—your school, friends, no one will know. Every paper’s custom-made to blend with your style, and we check it for originality, so it’s all yours, all discreet.
BIBLIOGRAPHY……………………………………………………………………………………………………………..
No way—our papers are 100% human-crafted. Our writers are real pros with degrees, bringing creativity and expertise AI can’t match. Every piece is original, checked for plagiarism, and tailored to your needs by a skilled human, not a machine.
CHAPTER 5
We’re the best because our writers are degree-holding experts—Bachelor’s to Ph.D.—who nail any topic. We obsess over quality, using tools to ensure perfection, and offer free revisions to guarantee you’re thrilled with the result, even on tight deadlines.
CHAPTER 1
My mum always said to me: “An apple a day to keep any physician at bay”. Originating from a typical Asian family, there are only 2 things my parents are overly concerned about: health and education. Although I’ve failed to comply my mums words; as for all my life I’ve despised fruits and vegetables and consumed mostly meat. I’ve neglected all the beneficial nutrients that fruits and vegetables provide for us and only ate what’s more appetising-meat. The only semi-appetising fruit I can handle is apples and through this EE, I’ve finally gotten a chance to investigate just how much nutrients apples really provide for us and whether it’s worthwhile eating.
Our writers are top-tier—university grads, many with Master’s degrees, who’ve passed tough tests to join us. They’re ready for any essay, working with you to hit your deadlines and grading standards with ease and professionalism.
Apple is usually a round, red, yellow or green edible fruit in a small tree (Malus Sylvestris) of the rose family. An apple contains nearly all the vitamins than humans need for their metabolic processes1. They are high in fiber- important for digestive health and boron- which promotes bone growth. Also minerals such as calcium, magnesium, sulfur, phosphorus and chlorine are found in all types of apples as well. It is said that one-tenth of our daily potassium needs are contained within one apple which helps to improve our muscle tone. More importantly one of the most known nutrients apple provide is Vitamin C (ascorbic acid) and is one of the most effective antioxidants, which help to grow and repair tissues in all parts of our bodies, for example healing a cut wound. Without enough Vitamin C present in our body, humans are likely to be exposed to more diseases, because harmful bacteria and exposure to harmful chemicals will all travel through our blood into our body while we have a wound open. Thus it is extremely crucial that there is sufficient Vitamin C present to repair the damaged tissues as quickly as possible to avoid unnecessary diseases. It has being discovered that one apple provides roughly 10% of our daily recommended allowance for Vitamin C; an apple weighing 138 grams contains roughly 6.3 milligrams of Vitamin C2.
After discovering the importance of Vitamin C, which every human necessitates. I then questioned the sugar content contained in apples. As we all know sugar is a type of carbohydrate. When carbohydrates are digested and sent into the blood stream they become sugars called glucose. Glucose provides energy when then the cells in our body will absorb. Consuming too much sugar it will cause our body to be resilient to insulin, which is a hormone that allows glucose to enter the cells from the bloodstream and controls the cells to burn glucose instead of fat, thus eating too much sugar will damage this process and will cause many diseases.
This ultimately lead me to think about how to eat an apple. I personally prefer eating soft foods which I can easily sink my teeth into rather than foods that are crunchy and hard. Knowing the fact that heating foods will cause it to become more ripe and soft, and also being the impatient man as I am I also questioned whether the amount of time when heating had an effect on Vitamin C and sugar content in apples. Thus I intend to find out how different heating temperatures of water and time affect the abundance of Vitamin C and sugar levels in apples.
1.1 Research Question
Always! We start from scratch—no copying, no AI—just pure, human-written work with solid research and citations. You can even get a plagiarism report to confirm it’s 95%+ unique, ready for worry-free submission.
“To what extent does the different temperature of water (50°C, 70°C and 100°C) and time (3min and 6min) that the apple (Red Delicious) is exposed to, affect the Vitamin C (ascorbic acid) and sugar content in Red Delicious, by measuring the concentration of Vitamin C and sugar still remaining in g per 100g of mass?”
(e.g. exposing the apple in 50°C for 3 and 6min. Then exposing the apple in 70°C for 3min and 6min etc.)
1.2 Background Information
Ascorbic Acid
You bet! From APA to IEEE, our writers nail every style with precision. Give us your guidelines, and we’ll craft a paper that fits your academic standards perfectly, no sweat.
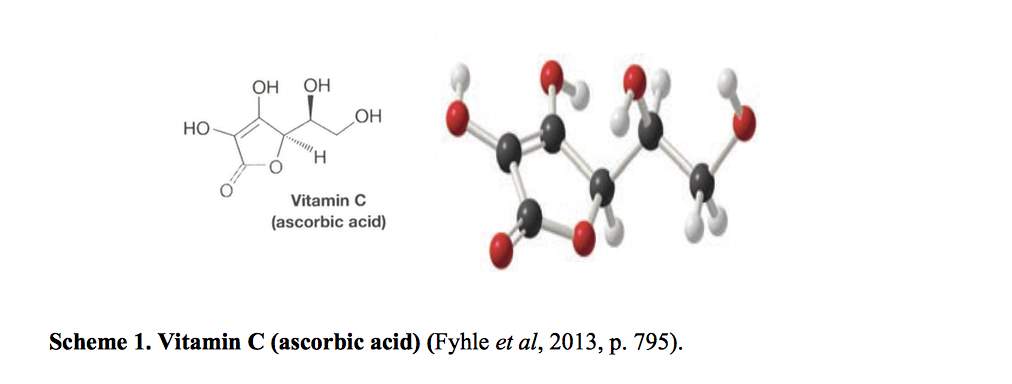 Ascorbic acid (C6H8O6) is a colourless and odourless crystalline substance with a molar mass of 176.12g/mol and is slightly sour in taste. It falls within the class of enolic lactones of glyculosonic acids. The most important member of this class is L-ascorbic acid. (Addy et al, p.200) L-ascorbic acid (C6H8O6) is the trivial name of Vitamin C. The chemical name for ascorbic acid is 2-oxo-L-threo-hexono-1,4- lactone-2,3-enediol (Naidu, 2003).
Ascorbic acid (C6H8O6) is a colourless and odourless crystalline substance with a molar mass of 176.12g/mol and is slightly sour in taste. It falls within the class of enolic lactones of glyculosonic acids. The most important member of this class is L-ascorbic acid. (Addy et al, p.200) L-ascorbic acid (C6H8O6) is the trivial name of Vitamin C. The chemical name for ascorbic acid is 2-oxo-L-threo-hexono-1,4- lactone-2,3-enediol (Naidu, 2003).
L-ascorbic acid is a co-factor for hydroxylases and monooxygenase enzymes involved in the synthesis of collagen. Collagen acts like ‘glue’ which helps to hold the cells within our body together resulting in the promotion of skin strength and elasticity, making our skin more durable to cuts.
Humans cannot synthesize ascorbic acid themselves as being a water soluble compound C vitamin is easily absorbed but it is not stored in the body (Naidu, 2003).
The average daily intake level that is sufficient to meet the nutritional requirement of ascorbic acid or recommended dietary allowances (RDA) for adults (>19 yr.) are 90 mg/day for men and 75 mg/day for women (Naidu, 2003).
Yep! Use our chat feature to tweak instructions or add details anytime—even after your writer’s started. They’ll adjust on the fly to keep your essay on point.
The stability of ascorbic acid decreases with increase in pH. Therefore high pH substances such as baking soda has a detrimental effect on the destruction of Vitamin C., but is fairly stable in weak acidic solutions. Also ascorbic acid content decreases gradually during storage especially at temperature above 0°C (Oyetade et al, 2012). The average half-life of ascorbic acid is between 16 and 20 days.
Ascorbic acid is reversibly oxidized to dehydroascorbic acid.
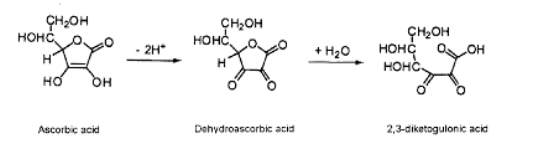 Further oxidation of dehydroascorbic acid leads to the irreversible formation of 2,3-diketogulonic acid.
Further oxidation of dehydroascorbic acid leads to the irreversible formation of 2,3-diketogulonic acid.
Easy—place your order online, and your writer dives in. Check drafts or updates as you go, then download the final paper from your account. Pay only when you’re happy—simple and affordable!
Scheme 3. Oxidation of Dehydroascorbic acid to 2,3-diketogulonic acid
Super fast! Our writers can deliver a quality essay in 24 hours if you’re in a pinch. Pick your deadline—standard is 10 days, but we’ll hustle for rush jobs without skimping.
Fructose and Glucose:
 Fructose and glucose are the simplest form of carbohydrates, found in many plants and fruits. They are monomers of sucrose, or are the simple monosaccharides, but when bonded they form the disaccharide of sucrose.
Fructose and glucose are the simplest form of carbohydrates, found in many plants and fruits. They are monomers of sucrose, or are the simple monosaccharides, but when bonded they form the disaccharide of sucrose.
The name mono (one) saccharides (sugar), means that fructose and glucose only contain one sugar group, thus they cannot be broken down any further.
Definitely! From astrophysics to literary theory, our advanced-degree writers thrive on tough topics. They’ll research deeply and deliver a clear, sharp paper that meets your level—high school to Ph.D.
All types of carbohydrates are directly absorbed directly into our blood during digestion and different subtype of carbohydrates has different effects on the body depending on the structure and source (Berg CM, et al). The chemical structure will affect the rate of absorption of the carbohydrate molecules, meanwhile the source of where the carbohydrates came from will determine whether other nutrients are absorbed along with the carbohydrates.
Fructose has a unique texture and a tint of sweetness and has a different rate of digestion compared to glucose; which is the sugar that most of our ingested carbohydrate become when they are absorbed into the bloodstream (Bes-Rastrollo M, et al.). Carbohydrates are an important source of energy for the chemical metabolism in our bodies.
It is extremely controversial where debates of sugar being beneficial or harmful to our body, but in reality, both are correct. When fructose is absorbed through the intestine and enters the liver. In the liver it can then be converted to glucose derivatives and stored as glycogen which is a source of energy for strenuous activities. But too much fructose consumption, will be converted to fat as the liver can only store a limited amount of glycogen, thus are the cause for high-blood pressures and diabetes (Bray GA.)
It is considered that consumption of white sugar should not exceed more than 10% of daily energetic needs. Our body needs daily different carbohydrates, including fibers (Maser et al, 2009).
We tailor your paper to your rubric—structure, tone, everything. Our writers decode academic expectations, and editors polish it to perfection, ensuring it’s grade-ready.
Upload your draft, tell us your goals, and our editors will refine it—boosting arguments, fixing errors, and keeping your voice. You’ll get a polished paper that’s ready to shine.
Sure! Need ideas? We’ll pitch topics based on your subject and interests—catchy and doable. Pick one, and we’ll run with it, or tweak it together.
CHAPTER 2
2.0 THE METHODOLOGY
During my background study on this topic, I didn’t find any researchers or sites investigating 2 different independent variables at the same time. There are certain debates that having more than 1 independent variable will falsify the results of dependent variable, as its impossible to tell which independent variable is actually affecting the dependent variable. But I beg to disagree, as if the 2 independent variables are thought out correctly it’s possible to determine the effects of both independent variable.
For my research question with the 2 independent variables being:
Yes! If you need quick edits, our team can turn it around fast—hours, not days—tightening up your paper for last-minute perfection.
By first cooking the apple in 50°C water for 3min and 6min and determining the Vitamin C and sugar content of each apple sample I can use the data found from this to determine whether the amount of time have an effect on the dependent variables. As everything else is kept constant thus eliminating the independent variable of different temperatures.
To determine whether the increase in temperature affect the sugar and Vitamin C content, it can be done by comparing the results found from cooking the apple in 50°C for 3min, 70°C for 3min and 100°C for 3min; where once again eliminating the independent variable of the different time.
There are numerous ways of determining the Vitamin C content, such as through the use of spectrophotometer or redox titration. Due to my lack of knowledge about spectrophotometers I’ve opted redox titration for the determination of Vitamin C (University of Canterbury, n.d.).
As for sugar content, the sugar amount in the investigated apple samples can be evaluated using standardised sugar solutions. By using the sugar solution volume/density ratio standard graph, the mass of sugar in the investigated apple samples can be determined (University of Canterbury, n.d.).
Hypothesis:
If the temperature and time increases when cooking the apple, then the concentration of Vitamin C and sugar content within the apple will decrease, because heat causes the particles to move faster and increases its kinetic energy therefore increases the chances of collisions, thus increasing the intermolecular space between the particles and allowing the Vitamin C and sugar particles to move out of the apple easier.
Absolutely! We’ll draft an outline based on your topic so you can approve the plan before we write—keeps everything aligned from the start.
As the time increases the concentration of Vitamin C and sugar content will also decrease, since the water temperature will be higher than the room temperature, therefore the intermolecular space between the particles will be further apart than an apple under room temperature conditions; thus the longer time it’s in the water the higher chance of Vitamin C and sugar particles to escape through the larger gaps. Also the particles will not possess as much kinetic energy if taken out early, meaning that the particles will be moving less thus having less chance to escape the apple.
Null hypothesis:
If the temperature and time increases while cooking the apple, the vitamin C content within the apple will not change, due to the fact that the ascorbic acid will be trapped within the outer layers of the apple, as no matter what kinetic energy the particle contains, it will be stopped once it hits an obstacle.
2.1 Preparing the Samples
All apparatus, as well as the Red Delicious apples were rinsed with deionised water before conducting the experiment. The apples are then cut into 6 cubes each weighing 100g. Each cube were cut off the same part of the apple, to ensure than the Vitamin C and sugar content are equal, and the volume of the apple were also kept the same so ensure that when cooking it in same amount of water (1dm3) the amount of Vitamin C and sugar particles that escape will be the same due to the same surface area to volume ratio.
You bet! Need stats or charts? Our writers can crunch numbers and craft visuals, making your paper both sharp and professional.
Each of the apple pieces were cooking in:
This process was then repeated, but instead of cooking it under 3min it was cooking under 6min instead.
2.2 Determining the Vitamin C Content
2.2.1 Equation of Redox reaction between Ascorbic Acid and Iodine:
C6H8O6 (aq) + I2 (aq) —> 2I– (aq) + C6H6O6 (aq) +2H+ (aq)
for the proof of this equation refer to Appendix (5.2)
Instead of just using iodine solution (I2), the formation of iodine was created using iodate ions and iodide ions, as potassium iodate and potassium iodide ions are more stable, than iodine with the exposure to various of elements in the atmosphere. The potassium ions act as a spectator and does not take place within the reaction.
When iodate ions (IO3-) are added to an acidic solution containing iodide ions (I–), an oxidation-reduction reaction occurs:
We break it down—delivering each part on time with consistent quality. From proposals to final drafts, we’re with you all the way.
IO3– (aq) + 5I– (aq) +6H+ (aq) 3I2 (aq) +3H2O (l)
For proof of equation refer to Appendix (5.3)
Yep! Whether it’s UK, US, or Australian rules, we adapt your paper to fit your institution’s style and expectations perfectly.
It is the iodine formed by this reaction then oxidises the ascorbic acid to dehydroascorbic acid as the iodine is reduced to iodide ions:
C6H8O6 (aq) + I2 (aq) —> 2I– (aq) + C6H6O6 (aq) +2H+ (aq)
Iodine will continue to be reduced since ascorbic acid acts as a reducing agent, where when ascorbic acid is present, iodine will continue to be reduced to I-, due to the fact that ascorbic acid will continue to give up its electrons and push it towards iodine atoms. Until the moment when ascorbic acid’s electrons are all used up, that’s when the excess iodine is free to react with the indicator (starch in this case), forming the blue-black starch-iodine complex – indicating the end point of the titration.
2.2.2 Creating the Required Solutions (refer to Appendix5.4):
0.5% starch
We write every paper from scratch just for you, and we get how important it is for you to feel confident about its originality. That’s why we double-check every piece with our own in-house plagiarism software before sending it your way. This tool doesn’t just catch copy-pasted bits—it even spots paraphrased sections. Unlike well-known systems like Turnitin (used by most universities), we don’t store or report anything to public databases, so your check stays private and safe. We stand by our plagiarism-free guarantee to ensure your paper is totally unique. That said, while we can promise no plagiarism from open web sources or specific databases we check, no tech out there (except Turnitin itself) can scan every source Turnitin indexes. If you want that extra peace of mind, we recommend running your paper through WriteCheck (a Turnitin service) and sharing the report with us.
Potassium iodate solution (0.002 MOL)
Potassium iodide solution (0.6 MOL)
Hydrochloric acid (1 MOL)
20 cm3 Ascorbic acid
The moment you place your order, we jump into action to find the perfect writer for you. Usually, we’ve got someone lined up within an hour. Sometimes, though, it might take a few hours—or in rare cases, a few days—if we need someone super specialized. If no writers from your chosen category are free, we’ll suggest one from a lower category and refund the difference if you’d paid extra for that option. Want to keep tabs on things? You can always peek at your order’s status on your personal order page.
The amount and concentration of each of these solutions were kept constant to ensure the same mols of each solution was reacted, which is important as the amount of Vitamin C content will be determined with mol ratios between potassium iodate and ascorbic acid. Also, for a reaction to occur the KIO3 particles need to collide with KI, ascorbic acid and also starch; thus too much or too little of any solution (excluding KIO3)will hinder KIO3 particle’s chance to collide and react with the required solutions, thus will falsify the results of the titres due to the fact that the results will also be affect by the concentration and amount of solutions used.
2.2.3 Vitamin C extraction:
The 6 pieces of apple after cooking them, were then taken out and was grinded using a pestle and mortar. After no more juice can be extracted out the apple piece was taken out and the juice was mixed with 30cm3 of distilled water. The solution was then poured into a volumetric flask and distilled water was added until the total volume hit 100 cm3.
2.2.4 Titration:
20ml of ascorbic acid was transferred into a conical flask, with the addition of 5cm3 HCl, potassium iodide (KI) and 1 cm3 starch indicator. The conical flask was then placed under the burette which was filled with 50cm3 of KIO3 (potassium iodate). The end point was the titration was at the point where a blue-black colour change occurred, and the amount of KIO3 was recorded when this colour change occurred. This experiment was repeated 3 times, to ensure the average titre of KIO3 were within (0.1 cm3).
2.3 Determining the Sugar Content
2.3.1 Creating standard sugar solution volume/density ratio graph
By first determining that sugar solution(fructose/glucose) is in aqueous state and sugar under room temperature it’s solid state, the conversion of 1cm3 of sugar volume is equal to 0.85g of sugar mass. Also considering that 1cm3 of distilled water is equivalent to 1g.
Each of the 5cm3, 10cm3, 15cm3 (4.25g, 8.5g and 12.75g) of sugar is poured into a 100cm3 beaker, which was then filled with distilled water until the final volume hit 50cm3 (e.g. for 5cm3 sugar, 45cm3 of distilled water is added). The beaker was weighed before the solution was poured, so the mass of the solution can be determined. As the mass of beaker was subtracted from the total mass (solution + beaker). The density was then determined using the formula
=mv
(density equals mass divided by volume). After determining the density of each sugar concentration, the results were graphed with the independent variable (x-axis) being the amount of sugar (cm3) and the dependent variable (y-axis) being the density.
2.3.2 Calculating Sugar content in Apple Sample:
After no more juice can be extracted out the 100g apple piece was taken out and the juice was mixed with 30cm3 of distilled water. The solution was poured into a 100cm3 beaker and distilled water was added until the final solution hit 50cm3. Similarly, density can then be found (refer to 2.3.1) thus using the graph from 3.1.1(refer to graph 1 under results section) and comparing the density of the apple sample to the graph of standardized sugar solution (density vs amount of sugar), the sugar content can then be determined for the apple samples.
CHAPTER 3
3.1 Calculating Sugar Content:
Formulas to consider:
=mv
3.1.1 Creating standardized sugar solution graph:
5cm3 fructose (1cm3 sugar is equal to 0.85g)
5 x 0.85 = 4.25g
45cm3 distilled water (1cm3 water is equal to 1g)
45 x 1 = 45g
Total volume = 5 + 45 = 50cm3
Total mass = 4.25 + 45 = 49.25g
=49.2550
=
0.985 g/cm3
10cm3 fructose
10 x 0.85 = 8.5g
40cm3 distilled water
40 x 1 = 40g
Total volume = 10 + 40 = 50cm3
Total mass = 8.5 + 40 = 48.50g
=48.550
=
0.970 g/cm3
15cm3 fructose
15 x 0.85 = 12.75g
35cm3 distilled water
35 x 1 = 35g
Total volume = 15 + 35 = 50cm3
Total mass = 12.75 + 35 = 47.75g
=47.7550
=
0.955 g/cm3
Table 1. The density of different fructose solution (5,10,15cm3) and the mass of sugar.
| Amount of sugar (cm3) 0.05cm3 | Mass of solution -sugar + water (g) 0.01g | Density of solution (g/cm3) | Mass of Sugar (g) 0.01g |
| 5 | 49.25 | 0.985 | 4.25 |
| 10 | 48.50 | 0.970 | 8.5 |
| 15 | 47.75 | 0.955 | 12.75 |
Graph 1: Sugar solution volume : density ratio standard graph
Density (g/cm3)
3.1.2 Calculating the Density of Sugar Solution Within Each Apple Samples (100g) at Different Temperature and Time:
Using the same density formula:
=mv
Table 2: Density of different sugar content within the 100g apple samples under different temperature and time.
| Temperature (oC) | Time (min) | Volume (cm3) | Mass (g) | Density (g/cm3) |
| 50 | 3 | 50 | 48.21 | 0.964 |
| 50 | 6 | 50 | 48.50 | 0.970 |
| 70 | 3 | 50 | 48.82 | 0.976 |
| 70 | 6 | 50 | 49.01 | 0.980 |
| 100 | 3 | 50 | 49.20 | 0.984 |
| 100 | 6 | 50 | 49.60 | 0.992 |
By comparing the density of the apple sample to the graph of standardized sugar solution (graph 1), the sugar content can then be determined for the apple samples.
Density (g/cm3)
This illustrates that at 0.964 g/cm3 density the sugar volume is 11.8cm3, for the apple sample that was cooked in 50oC water for 3min. This process was repeated for all the other conditions and the results are tabulated.
Table 3: Comparing the density to graph 1 to determine the sugar mass (g) still containing within 100g of apple sample under each condition.
| Temperature (oC) | Time (min) | Density (g/cm3) | Sugar Volume (cm3) | Sugar Mass (g) |
| 50 | 3 | 0.964 | 11.80 | 10.03 |
| 50 | 6 | 0.970 | 9.64 | 8.19 |
| 70 | 3 | 0.976 | 7.49 | 6.37 |
| 70 | 6 | 0.980 | 6.32 | 5.37 |
| 100 | 3 | 0.984 | 5.12 | 4.35 |
| 100 | 6 | 0.992 | 2.86 | 2.4 |
Remembering that 1cm3 fructose is equivalent to 0.85g in mass.
e.g. 11.8 x 0.85 = 10.03g of sugar
Graph 2: Comparison of Sugar Amount in 100g Apple Sample
A line of regression is drawn for the temperatures in 3min, to eliminate any outlier results, thus getting a more accurate reading for the sugar mass under that condition.
Amount of Sugar (g)
Temperature (oC)
The results on this graph illustrates that as temperature increases when time is kept constant, the amount of sugar in the sample decreases. At each of the same temperature but different time, the apple that’s cooked for longer had less sugar content. Also, as time increases, the black line transitions down from the y axis, illustrating that if the apples were cooked longer, the amount of sugar content will decrease. The 2 lines are parallel having the same gradient, meaning that the sugar content with increasing time will always have less content under the same temperature, as the 2 times will never intersect with the same gradient, therefore the orange line will always be below the black line. It can also be safe to say that for temperature <50oC the sugar content will be greater than the sugar content at 50oC and for temperature >100oC the sugar content will be less than the sugar content at 100oC. This result also illustrates, from ascending order of sugar content still remaining in 100g of apple:
100oC (6min) < 100oC (3min) < 70oC (6min) < 70oC (3min) < 50oC (6min) < 50oC (6min). This also shows, from the graph that temperature affects the sugar content more than time, because 100oC (3min) had less sugar content than 70oC (6min); and 70oC (3min) < 50oC (6min). Since from the result it’s safe to say that the increase in time and temperature will result in a decrease in sugar content, therefore comparing the higher temperature with less time and lower temperature with more time showed that higher temperature caused the sugar content to decrease more. But this finding cannot generalise that the increase in temperature will affect sugar content more than time, as the values of time and temperature was chosen randomly and may not have a direct correlation (e.g. if it was 3min and 8min, and the temperatures were still the same, then the results will be different).
3.2 Calculating Vitamin C Content:
Table 4: average titre (cm3) of KIO3 (0.002MOL) used for each apple sample under these conditions.
| Temperature (oC) | Time (min) | Titre 1 (cm3) | Titre 2 (cm3) | Titre 3 (cm3) | Average Titre (cm3) |
| 50 | 3 | 1.34 | 1.33 | 1.34 | 1.34 |
| 50 | 6 | 1.12 | 1.10 | 1.13 | 1.12 |
| 70 | 3 | 1.05 | 1.03 | 1.03 | 1.04 |
| 70 | 6 | 1.01 | 0.99 | 0.98 | 0.99 |
| 100 | 3 | 0.92 | 0.93 | 0.92 | 0.92 |
| 100 | 6 | 0.81 | 0.80 | 0.80 | 0.80 |
Reactions to Consider:
For the apple sample undergoing 50oC of water for 3min:
Concentration of KIO3 (0.002mol per 1dm3), and knowing that:
1 dm3 = 1000 cm3
0.0021000
= 0.000002 MOL (per 1 cm3)
Average titre of KIO3 : 1.34 cm3
n
=CV
(mols = concentration x volume)
1.34 x 0.000002 = 0.00000268 mol of KIO3
Referring to reaction 1.
1 IO3– : 3 I2
1 mol of IO3– is equivalent to 3 mol of I2
0.00000268 x 3 = 0.00000804 mol of I2
Referring to reaction 2.
1 I2 : 1 C6H8O6
Mol ratio is 1:1
0.00000804 x 1 = 0.00000804 mol of C6H8O6
M (C6H8O6) = 176g
Mass = molar mass x mol
0.00000804 x 176 = 0.001415g (1.415 mg) of ascorbic acid in 20 cm3 of ascorbic solution.
The total ascorbic acid of 100g apple sample was within 100cm3 of ascorbic solution, and the calculation was only for 20cm3.
20 : 100 = 1 : 5 ratio
1.415 x 5 = 7.075mg per 100g of apple
Repeating the process for each condition:
Table 5: amount of Vitamin C (mg per 100g) of apple in each condition
| Temperature (oC) | Time (min) | Amount of Vitamin C (mg per 100g) |
| 50 | 3 | 7.075 |
| 50 | 6 | 5.966 |
| 70 | 3 | 5.438 |
| 70 | 6 | 5.174 |
| 100 | 3 | 4.858 |
| 100 | 6 | 4.224 |

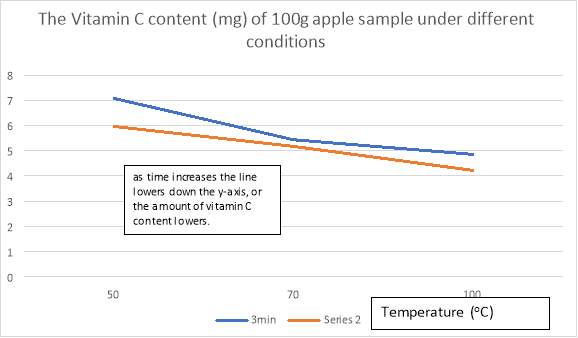 Graph 3: The Vitamin C content (mg) of 100g apple sample under different conditions.
Graph 3: The Vitamin C content (mg) of 100g apple sample under different conditions.
A line of regression is drawn for the temperatures in 3min, to eliminate any outlier results, thus getting a more accurate reading for the Vitamin C content under those conditions.
Amount of Vitamin C (mg)
This graph illustrates that the increase in temperature will reduce the amount of Vitamin C content in apples as shown either through the blue line or the orange line. Similarly, as time increases the Vitamin C content will also decrease, through comparing the results found with same temperature but different times. Following the trend, it can be safe to say that for temperature <50oC the apples will contain more vitamin C, and for temperature >100oC the apples will contain minimal vitamin C content. Also, as time increases the black line transitions down from the y axis, illustrating that if the apples were cooked longer the amount of vitamin C content will again, decrease. The black and orange lines are parallel, meaning that the black line will never intersect with the orange line as they have the same gradient, which is safe to assume that an increase in time will always result in less vitamin C content under the same temperature.
3.3 Calculating the vitamin C to Sugar content ratio
The calculations from 3.1 and 3.2 helped me to determine the amount of sugar and vitamin C content left, but I wanted to investigate the correlation between Vitamin C and sugar content and how it is affected with an increase in temperature and time; thus determining just how much sugar is within 1 mg of vitamin C, thus finding out the ratio:
Table 6: The Sugar : Vitamin C ratio in 100g apple sample under each conditions.
| Temperature (oC) | Time (min) | Sugar Mass (mg) | Vitamin C content (mg) | Sugar : Vitamin C ratio (mg : mg) |
| 50 | 3 | 10030 | 7.075 | 10030 : 7.075 |
You Want The Best Grades and That’s What We Deliver
Our top essay writers are handpicked for their degree qualification, talent and freelance know-how. Each one brings deep expertise in their chosen subjects and a solid track record in academic writing.
We offer the lowest possible pricing for each research paper while still providing the best writers;no compromise on quality. Our costs are fair and reasonable to college students compared to other custom writing services.
You’ll never get a paper from us with plagiarism or that robotic AI feel. We carefully research, write, cite and check every final draft before sending it your way.